DSLETCAS# 75644-90-5 |
- Valproic acid sodium salt (Sodium valproate)
Catalog No.:BCC2156
CAS No.:1069-66-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Panobinostat (LBH589)
Catalog No.:BCC3601
CAS No.:404950-80-7
- Belinostat (PXD101)
Catalog No.:BCC2153
CAS No.:414864-00-9
- Trichostatin A (TSA)
Catalog No.:BCC3605
CAS No.:58880-19-6
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 75644-90-5 | SDF | Download SDF |
| PubChem ID | 107847 | Appearance | Powder |
| Formula | C33H46N6O10 | M.Wt | 686.9 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 0.50 mg/ml in sterile water | ||
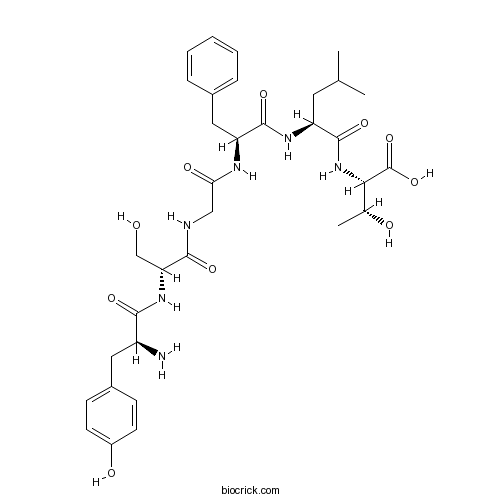
| Chemical Name | (2S,3R)-2-[[(2S)-2-[[(2S)-2-[[2-[[(2R)-2-[[(2S)-2-amino-3-(4-hydroxyphenyl)propanoyl]amino]-3-hydroxypropanoyl]amino]acetyl]amino]-3-phenylpropanoyl]amino]-4-methylpentanoyl]amino]-3-hydroxybutanoic acid | ||
| SMILES | CC(C)CC(C(=O)NC(C(C)O)C(=O)O)NC(=O)C(CC1=CC=CC=C1)NC(=O)CNC(=O)C(CO)NC(=O)C(CC2=CC=C(C=C2)O)N | ||
| Standard InChIKey | PKSODCLCMBUCPW-LVNBQDLPSA-N | ||
| Standard InChI | InChI=1S/C33H46N6O10/c1-18(2)13-24(32(47)39-28(19(3)41)33(48)49)37-31(46)25(15-20-7-5-4-6-8-20)36-27(43)16-35-30(45)26(17-40)38-29(44)23(34)14-21-9-11-22(42)12-10-21/h4-12,18-19,23-26,28,40-42H,13-17,34H2,1-3H3,(H,35,45)(H,36,43)(H,37,46)(H,38,44)(H,39,47)(H,48,49)/t19-,23+,24+,25+,26-,28+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | δ Opioid receptor agonist. |

DSLET Dilution Calculator

DSLET Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.4558 mL | 7.2791 mL | 14.5582 mL | 29.1163 mL | 36.3954 mL |
| 5 mM | 0.2912 mL | 1.4558 mL | 2.9116 mL | 5.8233 mL | 7.2791 mL |
| 10 mM | 0.1456 mL | 0.7279 mL | 1.4558 mL | 2.9116 mL | 3.6395 mL |
| 50 mM | 0.0291 mL | 0.1456 mL | 0.2912 mL | 0.5823 mL | 0.7279 mL |
| 100 mM | 0.0146 mL | 0.0728 mL | 0.1456 mL | 0.2912 mL | 0.364 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Chalcostrobamine
Catalog No.:BCN1900
CAS No.:75638-72-1
- Knightolamine
Catalog No.:BCN1912
CAS No.:75638-70-9
- Oncrasin 1
Catalog No.:BCC2390
CAS No.:75629-57-1
- Gomisin K1
Catalog No.:BCN7030
CAS No.:75629-20-8
- Moracenin B
Catalog No.:BCC8341
CAS No.:75629-19-5
- CHAPS
Catalog No.:BCC1476
CAS No.:75621-03-3
- (-)-Usnic acid
Catalog No.:BCN4306
CAS No.:7562-61-0
- (S)-(+)-α-Methylhistamine dihydrobromide
Catalog No.:BCC6700
CAS No.:75614-93-6
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Methyl 3,4,5-trimethoxycinnamate
Catalog No.:BCN4589
CAS No.:7560-49-8
- Kaerophyllin
Catalog No.:BCN4304
CAS No.:75590-33-9
- alpha-Tocopherolquinone
Catalog No.:BCN4305
CAS No.:7559-04-8
- Boc-D-Asn-OH
Catalog No.:BCC3362
CAS No.:75647-01-7
- Strobamine
Catalog No.:BCN1943
CAS No.:75656-91-6
- 4-Acetyl-1,1-dimethylpiperazinium iodide
Catalog No.:BCC6616
CAS No.:75667-84-4
- 2,4-Dihydroxy-4,6-dimethoxydihydrochalcone
Catalog No.:BCN1363
CAS No.:75679-58-2
- Isradipine (Dynacirc)
Catalog No.:BCC3797
CAS No.:75695-93-1
- 5-Amino-2-methylindole
Catalog No.:BCC8731
CAS No.:7570-49-2
- Leflunomide
Catalog No.:BCC1256
CAS No.:75706-12-6
- Eupalinilide B
Catalog No.:BCN2521
CAS No.:757202-08-7
- Eupalinilide C
Catalog No.:BCN2522
CAS No.:757202-11-2
- Eupalinilide D
Catalog No.:BCN2523
CAS No.:757202-14-5
- 17-AAG (KOS953)
Catalog No.:BCC2121
CAS No.:75747-14-7
- Cedrusin
Catalog No.:BCN4307
CAS No.:75775-36-9
The effect of opioid agonists of delta-class DSLET, mu-class DAMGO, kappa-class U-69593 and an opioid antagonist, naloxone, on MTT activity of NALM-1 leukemic cells.[Pubmed:12481982]
Biomed Pharmacother. 2002 Nov;56(9):458-62.
The effects of synthetic agonists of delta-, mu-, kappa-opioid classes were studied on the proliferation of NALM-1 leukemic cells, using the MTT-test. Delta-opioid DSLET and mu-opioid DAMGO mildly and transiently decreased, in higher concentrations, the MTT-activity of NALM-1 cells after 6 h of treatment. The kappa-opioid agonist U-69593 mildly suppressed proliferation of NALM-1 cells after 48 h of treatment. Naloxone, an opioid receptor antagonist, mildly and transiently diminished MTT-activity of NALM-1 cells after 6 h of treatment. Treatment with opioid agonists, DAMGO, DSLET, U-69593, and an opioid antagonist naloxone for 6, 24, and 48 h, did not trigger DNA fragmentation, which was considered as a possible mechanism of action.
DSLET and ACTH(4-10) increase mitotic activity of hepatocytes and suppress antibody production.[Pubmed:12910277]
Bull Exp Biol Med. 2003 May;135(5):428-9.
The mitotic index of hepatocytes remained unchanged after 10 intraperitoneal injections of DSLET and ACTH(4-10) in doses of 0.5, 1.5, and 5 microg/kg, but increased after injection of these substances in doses of 50 and 150 microg/kg. DSLET in doses of 5, 50, and 150 microg/kg decreased the number of antibody-producing cells in the spleen. ACTH(4-10) possessed immunosuppressive activity not only in these doses, but also in a dose of 1.5 microg/kg. As differentiated from mitotic activity of hepatocytes, the degree of immunosuppression increased with increasing the dose of test peptides.
Affinity labeling of delta opioid receptors by an enkephalin-derivative alkylating agent, DSLET-Mal.[Pubmed:10558900]
Biochem Biophys Res Commun. 1999 Nov 19;265(2):513-9.
Opioid binding properties of Tyr-D-Ser-Gly-Phe-Leu-Thr-NH-NH-Gly-Mal (DSLET-Mal), a novel enkephalin-framed affinity label, was determined in rat brain membranes. In competition studies the ligand showed high affinity for the delta opioid sites, labelled by [(3)H][Ile(5,6)]deltorphin II (K(i) = 8 nM), whereas its binding to the mu ([(3)H]DAMGO) and kappa ([(3)H]EKC) sites was weaker. Preincubation of the rat brain membranes with DSLET-Mal at micromolar concentrations resulted in a wash-resistant and dose-dependent inhibition of the [(3)H][Ile(5,6)]deltorphin II binding sites (96% blocking at 10 microM concentration). Intracerebroventricular (ICV) administration of DSLET-Mal reduced the density of delta opioid receptors and had no effect on mu and kappa receptors, as determined by saturation binding studies. [Ile(5, 6)]deltorphin II-stimulated [(35)S]GTPgammaS binding was determined in membrane preparations of different brain areas of the ICV-treated animals. In both frontal cortex and hippocampus DSLET-Mal significantly decreased G protein activation by the delta agonist, having no effect on DAMGO stimulated [(35)S]GTPgammaS binding. DSLET-Mal had qualitatively similar effects on both receptor binding and G protein activation. These characteristics of the compound studied suggest that DSLET-Mal can serve as an affinity label for further studies of the delta-opioid receptors.
Autoradiographic comparison of [3H]DPDPE and [3H]DSLET binding: evidence for distinct delta 1 and delta 2 opioid receptor populations in rat brain.[Pubmed:8782867]
Brain Res. 1996 May 6;719(1-2):85-95.
The delta opioid ligands, [3H]DPDPE (delta 1) and [3H]DSLET (delta 2) were used in quantitative autoradiographic experiments to ascertain whether separate populations of delta opioid subtypes could be identified in rat brain. Densitometric image analysis showed a general similarity in delta 1 and delta 2 distributions. However, statistically significant differences in binding levels were observed in anatomically discrete regions. Examples of these regions and their delta 2/delta 1 ratio(s) are: dorsomedial hypothalamus (9.3), ventromedial hypothalamus (4.9), superior colliculis (2.7), medial division of bed nucleus stria terminalis (1.6-3.0), external cortex of the inferior colliculis (2.1), amygdaloid nuclei (1.5-2.1), cingulate cortex (1.8), CA1, CA2, and CA3 regions of Ammon's horn (1.6-2.0), dentate gyrus (1.7), laminar VI of the frontal, forelimb, hindlimb and parietal cortices (1.3-1.8), nucleus accumbens (1.4) and caudate/putamen (1.3). These findings provide evidence supporting the existence of distinct delta 1 and delta 2 opioid receptors.


