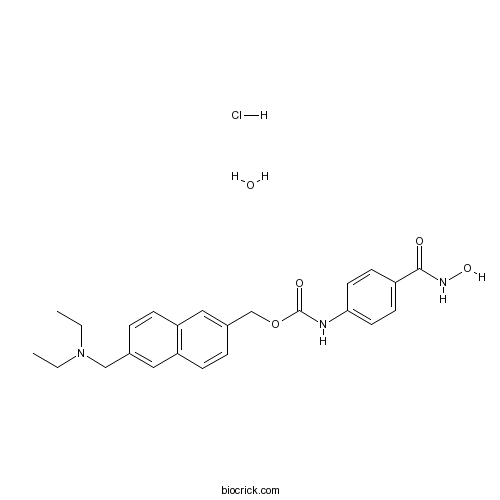
ITF2357 (Givinostat)HDAC inhibitor CAS# 732302-99-7 |
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- NSC 3852
Catalog No.:BCC2423
CAS No.:3565-26-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 732302-99-7 | SDF | Download SDF |
| PubChem ID | 9804991 | Appearance | Powder |
| Formula | C24H30ClN3O5 | M.Wt | 475.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Gavinostat hydrochloride monohydrate; ITF-2357 hydrochloride monohydrate | ||
| Solubility | DMSO : ≥ 100 mg/mL (210.10 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | [6-(diethylaminomethyl)naphthalen-2-yl]methyl N-[4-(hydroxycarbamoyl)phenyl]carbamate;hydrate;hydrochloride | ||
| SMILES | CCN(CC)CC1=CC2=C(C=C1)C=C(C=C2)COC(=O)NC3=CC=C(C=C3)C(=O)NO.O.Cl | ||
| Standard InChIKey | FKGKZBBDJSKCIS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C24H27N3O4.ClH.H2O/c1-3-27(4-2)15-17-5-7-21-14-18(6-8-20(21)13-17)16-31-24(29)25-22-11-9-19(10-12-22)23(28)26-30;;/h5-14,30H,3-4,15-16H2,1-2H3,(H,25,29)(H,26,28);1H;1H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Givinostat (ITF2357) is a potent inhibitor of HDAC for maize HD2, HD1B and HD1A with IC50 of 10 nM, 7.5 nM and 16 nM, respectively. | |||||
| Targets | HDAC | |||||
| IC50 | 7.5 to 16 nM | |||||
| Cell experiment: [1] | |
| Cell lines | PBMC cells |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 100 nM, 24 hours for hyperacetylation |
| Applications | To test the acetylation ability of ITF2357, rested PBMCs were preincubated with the inhibitor for 1 h at 37° C and then stimulated with LPS. After 3, 6, and 24 h, extracts of the cell pellets were made and the acetylated lysines were determined in total cellular extracts. After 3 h of incubation with LPS in the presence of ITF2357, hyperacetylation is clearly present. However, a greater duration of hyperacetylation up to 24 h was observed in cells exposed to ITF2357. |
| Animal experiment: [1] | |
| Animal models | BALB/C and C57BL/6 mice |
| Dosage form | Gavage, 5 mg/kg (BALB/C mice) Gavage, 1 or 10 mg/kg (C57BL/6 mice) |
| Application | Mice were given 100 µL water or ITF2357 (5 mg/kg) by gavage and, after 1 h, injected intravenously with 200 µg/mouse of ConA. Mice were bled 24 h later for evaluation of serum ALT levels. ITF2357 pretreatment reduced more than 80% of the ALT levels. A dose of 1 mg/kg ITF2357 was as effective as a dose of 10 mg/kg in reducing ConA hepatitis as measured by ALT levels. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Leoni F, Fossati G, Lewis E C, et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Molecular Medicine, 2005, 11(1-12): 1. | |

ITF2357 (Givinostat) Dilution Calculator

ITF2357 (Givinostat) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.101 mL | 10.5049 mL | 21.0097 mL | 42.0195 mL | 52.5243 mL |
| 5 mM | 0.4202 mL | 2.101 mL | 4.2019 mL | 8.4039 mL | 10.5049 mL |
| 10 mM | 0.2101 mL | 1.0505 mL | 2.101 mL | 4.2019 mL | 5.2524 mL |
| 50 mM | 0.042 mL | 0.2101 mL | 0.4202 mL | 0.8404 mL | 1.0505 mL |
| 100 mM | 0.021 mL | 0.105 mL | 0.2101 mL | 0.4202 mL | 0.5252 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
In models of arthritis, ITF2357, an HDACi, reduced joint swelling and cell influx into the joint cavity, improved the chondrocyte metabolic function, decreased production of proinflammatory cytokines, ameliorates the severity scores and prevented bone destruction.
Abstract
ITF2357, an inhibitor of class I and II HDAC, decreased renal disease, inflammatory cytokines and Th17 phenotype in NZB/W mice; while it increased the percentage of Tregs and Foxp3 acetylation.
Abstract
ITF2357 is an HDAC inhibitor that inhibits production of nitrite, TNFα and IFNγ in peritoneal macrophages and splenocytes. ITF2357 normalized STZ-induced hyperglycemia in mice returning serum nitrite levels to nondiabetic values, improving islet and increasing glucose clearance; while it increased islet cell viability, enhanced insulin secretion, inhibited MIP-1α and MIP-2 release, reduced nitric oxide production and decreased apoptosis rates in vitro.
Abstract
ITF2357, an HDAC inhibitor, has been evaluated for its efficacy on HIC-1 expression from latently infected cells and its effect on the surface expression of CXCR4 and CCR5.
Abstract
ITF2357, an HDAC inhibitor, was used to treatment patients with autoflammatory syndrome, including 1 patient with TRAPS, 3 patients with HIDS and 4 patients with Schnitzler syndrome, in a pilot study where ITF2357-induced partial response was only observed in patients with Schnitzler syndrome.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
ITF2357, also known as givinostat, is a potent inhibitor of both class I and class II histone deacetylase (HDAC) as well as a potent inhibitor of hematopoietic colony formation by JAKEV617F-bearing progenitor cells from chronic myeloproliferative neoplasms in vitro. Previous studies has shown that ITF2357 induces apoptosis of multiple myeloma (MM) and acute myelogenous leukemia (AML) cells following induction of p21 and down-modulation of Bcl-2 and Mcl-1 proteins and inhibits the production of pro-inflammatory cytokines (such as IL-1, IL-6, tumor necrosis factor (TNF)-α and interferon (IFN)-γ) by peripheral blood mononuclear cells as well as the production of IL-6 and vascular endothelium growth factor (VEGF) by mesenchymal stromal cells.
Reference
Katia Todoerti, Valentina Barbui, Olga Pedrini, Marta Linett, Gianluca Fossati, Paolo Mascagni, Alessandro Rambaldi, Antonino Neri, Martino Introna, Luigia Lombardi, and Josee Golay. Pleiotropi anti-myeloma activity of ITF2357: inhibition of interleukin-6 receptor signaling and repression of miR-19a and miR-19b. Haematologica 2010; 95(2): 260-269
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
Inhibition of the Autophagy Pathway Synergistically Potentiates the Cytotoxic Activity of Givinostat (ITF2357) on Human Glioblastoma Cancer Stem Cells.[Pubmed:27833530]
Front Mol Neurosci. 2016 Oct 27;9:107.
Increasing evidence highlighted the role of cancer stem cells (CSCs) in the development of tumor resistance to therapy, particularly in glioblastoma (GBM). Therefore, the development of new therapies, specifically directed against GBM CSCs, constitutes an important research avenue. Considering the extended range of cancer-related pathways modulated by histone acetylation/deacetylation processes, we studied the anti-proliferative and pro-apoptotic efficacy of givinostat (GVS), a pan-histone deacetylase inhibitor, on cell cultures enriched in CSCs, isolated from nine human GBMs. We report that GVS induced a significant reduction of viability and self-renewal ability in all GBM CSC cultures; conversely, GVS exposure did not cause a significant cytotoxic activity toward differentiated GBM cells and normal mesenchymal human stem cells. Analyzing the cellular and molecular mechanisms involved, we demonstrated that GVS affected CSC viability through the activation of programmed cell death pathways. In particular, a marked stimulation of macroautophagy was observed after GVS treatment. To understand the functional link between GVS treatment and autophagy activation, different genetic and pharmacological interfering strategies were used. We show that the up-regulation of the autophagy process, obtained by deprivation of growth factors, induced a reduction of CSC sensitivity to GVS, while the pharmacological inhibition of the autophagy pathway and the silencing of the key autophagy gene ATG7, increased the cell death rate induced by GVS. Altogether these findings suggest that autophagy represents a pro-survival mechanism activated by GBM CSCs to counteract the efficacy of the anti-proliferative activity of GVS. In conclusion, we demonstrate that GVS is a novel pharmacological tool able to target GBM CSC viability and its efficacy can be enhanced by autophagy inhibitory strategies.
The histone deacetylase inhibitor givinostat (ITF2357) exhibits potent anti-tumor activity against CRLF2-rearranged BCP-ALL.[Pubmed:28331226]
Leukemia. 2017 Nov;31(11):2365-2375.
Leukemias bearing CRLF2 and JAK2 gene alterations are characterized by aberrant JAK/STAT signaling and poor prognosis. The HDAC inhibitor givinostat/ITF2357 has been shown to exert anti-neoplastic activity against both systemic juvenile idiopathic arthritis and myeloproliferative neoplasms through inhibition of the JAK/STAT pathway. These findings led us to hypothesize that givinostat might also act against CRLF2-rearranged BCP-ALL, which lack effective therapies. Here, we found that givinostat inhibited proliferation and induced apoptosis of BCP-ALL CRLF2-rearranged cell lines, positive for exon 16 JAK2 mutations. Likewise, givinostat killed primary cells, but not their normal hematopoietic counterparts, from patients carrying CRLF2 rearrangements. At low doses, givinostat downregulated the expression of genes belonging to the JAK/STAT pathway and inhibited STAT5 phosphorylation. In vivo, givinostat significantly reduced engraftment of human blasts in patient-derived xenograft models of CRLF2-positive BCP-ALL. Importantly, givinostat killed ruxolitinib-resistant cells and potentiated the effect of current chemotherapy. Thus, givinostat in combination with conventional chemotherapy may represent an effective therapeutic option for these difficult-to-treat subsets of ALL. Lastly, the selective killing of cancer cells by givinostat may allow the design of reduced intensity regimens in CRLF2-rearranged Down syndrome-associated BCP-ALL patients with an overall benefit in terms of both toxicity and related complications.
Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat).[Pubmed:21365126]
Mol Med. 2011 May-Jun;17(5-6):353-62.
ITF2357 (Givinostat) is a histone deacetylase inhibitor with antiinflammatory properties at low nanomolar concentrations. We report here a phase I safety and pharmacokinetics trial in healthy males administered 50, 100, 200, 400 or 600 mg orally. After 50 mg, mean maximal plasma concentrations reached 104 nmol/L 2 h after dosing, with a half-life of 6.9 h. After 100 mg, maximal concentration reached 199 nmol/L at 2.1 h with a half-life of 6.0 h. Repeat doses for 7 consecutive days of 50, 100 or 200 mg resulted in nearly the same kinetics. There were no serious adverse effects (AEs) and no organ toxicities. However, there was a dose-dependent but transient fall in platelets. After 7 daily doses of 50 or 100 mg, the mean decrease in platelets of 17 and 25% was not statistically significant and returned to baseline within 14 d. Blood removed from the subjects after oral dosing was cultured ex vivo with endotoxin, and the release of tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6, IL-1Ra, interferon (IFN)-gamma and IL-10 was determined. Maximal reduction in IL-1beta, TNFalpha, IL-6 and IFNgamma was observed 4 h after dosing but returned to baseline at 12 h. There was no significant reduction in IL-1Ra or IL-10. With daily dosing, the fall in cytokine production in blood cultures observed on day 7 was nearly the same as that of the first day. We conclude that dosing of 50 or 100 mg ITF2357 is safe in healthy humans and transiently but repeatedly reduces the production of proinflammatory cytokines without affecting production of antiinflammatory cytokines.


