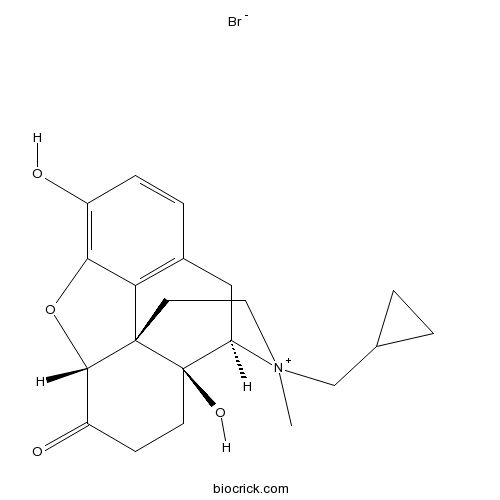
Methylnaltrexone BromideAntagonist of peripherally acting μ-opioid CAS# 73232-52-7 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73232-52-7 | SDF | Download SDF |
| PubChem ID | 5361917 | Appearance | Powder |
| Formula | C21H26BrNO4 | M.Wt | 436.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 31 mg/mL (71.05 mM); | ||
| Chemical Name | (4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-dihydroxy-3-methyl-2,4,5,6,7a,13-hexahydro-1H-4,12-methanobenzofuro[3,2-e]isoquinoline-3-ium-7-one;bromide | ||
| SMILES | [Br-].C[N+]1(CC[C@]23[C@H]4Oc5c(O)ccc(C[C@@H]1[C@]2(O)CCC4=O)c35)CC6CC6 | ||
| Standard InChIKey | IFGIYSGOEZJNBE-KNLJMPJLSA-N | ||
| Standard InChI | InChI=1S/C21H25NO4.BrH/c1-22(11-12-2-3-12)9-8-20-17-13-4-5-14(23)18(17)26-19(20)15(24)6-7-21(20,25)16(22)10-13;/h4-5,12,16,19,25H,2-3,6-11H2,1H3;1H/t16-,19+,20+,21-,22?;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Methylnaltrexone (Bromide) is a peripheral-acting opioid receptor antagonist that acts on the gastrointestinal tract to decrease opioid-induced constipation.In Vivo:Methylnaltrexone bromide (1 and 10 mg/kg, s.c.) has no effect on tyrosine-induced analgesia. Intracerebroventricular injection of Methylnaltrexone bromide (1 and 10 mg/rat) effectively blocks tyrosine-induced analgesia[1]. Methylnaltrexone bromide (0.01-10.00 mg/kg), does not significantly influence retention latencies of either shocked or unshocked mice. Further, Methylnaltrexone bromide (0.1, 1.0, or 10.0 mg/kg) does not block the retention impairment produced by concurrently administered morphine (3.0 mg/kg) or beta-endorphin (0.1 mg/kg)[2]. References: | |||||

Methylnaltrexone Bromide Dilution Calculator

Methylnaltrexone Bromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2918 mL | 11.459 mL | 22.9179 mL | 45.8358 mL | 57.2948 mL |
| 5 mM | 0.4584 mL | 2.2918 mL | 4.5836 mL | 9.1672 mL | 11.459 mL |
| 10 mM | 0.2292 mL | 1.1459 mL | 2.2918 mL | 4.5836 mL | 5.7295 mL |
| 50 mM | 0.0458 mL | 0.2292 mL | 0.4584 mL | 0.9167 mL | 1.1459 mL |
| 100 mM | 0.0229 mL | 0.1146 mL | 0.2292 mL | 0.4584 mL | 0.5729 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Methylnaltrexone Bromide is an antagonist of mu-opioid receptors (MOR) with IC50 value of 75nM.
MOR are a class of opioid receptors with high affinity for enkephalins and beta-endorphin but low affinity for dynorphins. Activation of MOR in the intestinal tract inhibits peristaltic action which causes constipation [1].
In patients with constipation, Methylnaltrexone Bromide plus standard care relieved the constipation symptoms with less time (38 days) and improved quality life of 0.02 years or 8.8 days [1]. Methylnaltrexone is a novel MOR antagonist, which does not pass the blood brain barrier. In the first two weeks of usage, Methylnaltrexone Bromide caused laxation in less than 24 hours for at least half of those patients without impairing pain control or causing serious adverse effects. In phase II studies, all 22 patients had fast laxation [2].
References:
[1]. Earnshaw SR, Klok RM, Iyer S, et al. Methylnaltrexone bromide for the treatment of opioid-induced constipation in patients with advanced illness--a cost-effectiveness analysis. Aliment Pharmacol Ther, 2010, 31(8): 911-921.
[2]. Bader S, Jaroslawski K, Blum HE, et al. Opioid-induced constipation in advanced illness: safety and efficacy of methylnaltrexone bromide. Clin Med Insights Oncol, 2011, 5: 201-211.
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
- SCH 50911
Catalog No.:BCC5692
CAS No.:733717-87-8
Opioid-induced constipation in advanced illness: safety and efficacy of methylnaltrexone bromide.[Pubmed:21836816]
Clin Med Insights Oncol. 2011;5:201-11.
Constipation, one of the major side effects of opiates used in palliative care, can impair patients' quality of life to a point where it prevents sufficient pain control. Methylnaltrexone is a novel mu-receptor antagonist, which does not pass the blood brain barrier. It is licensed to treat opiate induced constipation for patients with advanced diseases. This review article presents an overview of pharmacology and safety of its application, evidence of its efficacy and economic aspects of its use in clinical practice. Available data are limited but strongly suggest that methylnaltrexone causes laxation in less than 24 hours for at least half of those patients over the first two weeks of usage without impairing pain control or causing serious adverse effects. To avoid danger of gastrointestinal perforation it is contraindicated for patients at risk for that complication. More research is needed to evaluate its long-term efficacy and economic impact.
Methylnaltrexone bromide: research update of pharmacokinetics following parenteral administration.[Pubmed:21222554]
Expert Opin Drug Metab Toxicol. 2011 Feb;7(2):227-35.
INTRODUCTION: Opioid-induced constipation is a major side effect of the use of opioid pain medications in a palliative care population. At present, the only approved treatment for opioid-induced constipation is Methylnaltrexone Bromide subcutaneous injection. Methylnaltrexone is a peripherally restricted opioid antagonist with mu-opioid receptor selectivity that can reduce opioid activity in peripheral organs such as the gastrointestinal tract while sparing the pain relief afforded by the pain medications. AREAS COVERED: This article addresses the pharmacokinetics of parenterally administered methylnaltrexone, including the studies in humans that form the basis for our understanding, and information on the disposition, metabolism and elimination of the drug. From this review, the reader will gain an understanding of the body's handling of methylnaltrexone following intravenous or subcutaneous administration. EXPERT OPINION: Studies conducted to date indicate that methylnaltrexone has high bioavailability after subcutaneous administration at therapeutic dose levels, a terminal half-life of approximately 8 - 9 h, minimal metabolism, elimination involving renal and non-renal routes, and a limited potential for drug-drug interactions. Combined with high efficacy and good tolerability, the predictable pharmacokinetic behavior of methylnaltrexone facilitates its successful utilization in clinical practice for the treatment of opioid-induced constipation in patients with advanced medical illness.
Managing opioid-induced constipation in advanced illness: focus on methylnaltrexone bromide.[Pubmed:20234787]
Ther Clin Risk Manag. 2010 Mar 3;6:77-82.
Constipation is a common symptom in palliative care patients which can generate considerable suffering. There is uncertainty about the choice of treatment options from varying recommendations for management of constipation and a varying clinical practice in palliative care settings. The purpose of the review was to evaluate the current recommendations of therapy guidelines for the management of opioid-induced constipation in palliative care patients with a focus on Methylnaltrexone Bromide. Recent findings in the literature and related information on the opioid-induced gastrointestinal disorders in patients with advanced illness, as well as information on the opioid-antagonist methylnaltrexone, are discussed. Knowledge of the role of definitions, the causes of constipation and the pathophysiology of opioid-induced constipation must be given high priority in the treatment of patients receiving opioids. Diagnosis and therapy of constipation, therefore, should relate to findings in clinical investigation. Opioid-induced constipation and its adequate treatment is an important issue for patients with advanced illness and also poses therapeutic challenge for clinicians in daily routine. Methylnaltrexone Bromide may represent an important therapeutic option for palliative care patients who are suffering from opioid-induced constipation with failure of conventional prophylactic oral laxative treatment.


