BAY 61-3606Syk Inhibitor CAS# 732983-37-8 |
- R788 disodium
Catalog No.:BCC3695
CAS No.:1025687-58-4
- PRT062607 Hydrochloride
Catalog No.:BCC1869
CAS No.:1370261-97-4
- BAY 61-3606 dihydrochloride
Catalog No.:BCC1407
CAS No.:648903-57-5
- R406 (free base)
Catalog No.:BCC2553
CAS No.:841290-80-0
- R406
Catalog No.:BCC3876
CAS No.:841290-81-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 732983-37-8 | SDF | Download SDF |
| PubChem ID | 10200390 | Appearance | Powder |
| Formula | C20H18N6O3 | M.Wt | 390.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 5 mg/mL (12.81 mM; ultrasonic and warming and heat to 80°C) | ||
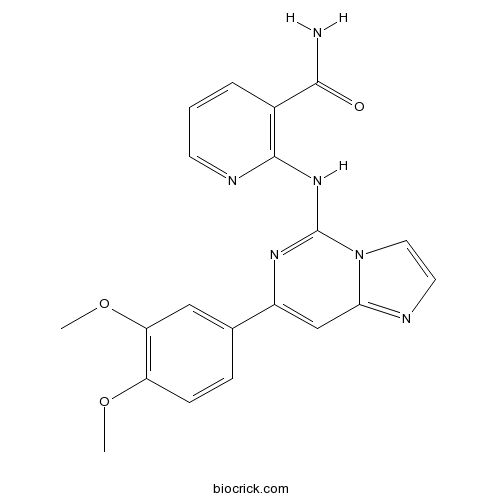
| Chemical Name | 2-[[7-(3,4-dimethoxyphenyl)imidazo[1,2-c]pyrimidin-5-yl]amino]pyridine-3-carboxamide | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC3=NC=CN3C(=N2)NC4=C(C=CC=N4)C(=O)N)OC | ||
| Standard InChIKey | JWQOJVOKBAAAAR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H18N6O3/c1-28-15-6-5-12(10-16(15)29-2)14-11-17-22-8-9-26(17)20(24-14)25-19-13(18(21)27)4-3-7-23-19/h3-11H,1-2H3,(H2,21,27)(H,23,24,25) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | BAY 61-3606 is a potent, ATP-competitive, reversible, and highly selective inhibitor of Syk tyrosine kinase activity (Ki= 7.5 nM) with no inhibitory effect against Btk, Fyn, Itk, Lyn, and Src.
IC50 value: 7.5 nM (Ki) [1]
Target: Syk
in vitro: BAY 61-3606 inhibited not only degranulation (IC50 values between 5 and 46 nM) but also lipid mediator and cytokine synthesis in mast cells. BAY 61-3606 was highly efficacious in basophils obtained from healthy human subjects (IC50 = 10 nM) and seems to be at least as potent in basophils obtained from atopic (high serum IgE) subjects (IC50 = 8.1 nM). B cell receptor activation and receptors for Fc portion of IgG signaling in eosinophils and monocytes were also potently suppressed by BAY 61-3606 [1]. We identified BAY61-3606 as an inhibitor of proliferation in colorectal cancer cells expressing mutant forms of K-RAS, but not in isogenic cells expressing wild-type K-RAS. In addition to its anti-proliferative effects in mutant cells, BAY61-3606 exhibited a distinct biological property in wild-type cells in that it conferred sensitivity to inhibition of RAF. In this context, BAY61-3606 acted by inhibiting MAP4K2 (GCK), which normally activates NFκβ signaling in wild-type cells in response to inhibition of RAF [2].
in vivo: Oral administration of BAY 61-3606 to rats significantly suppressed antigen-induced passive cutaneous anaphylactic reaction, bronchoconstriction, and bronchial edema at 3 mg/kg. Furthermore, BAY 61-3606 attenuated antigen-induced airway inflammation in rats [1]. References: | |||||

BAY 61-3606 Dilution Calculator

BAY 61-3606 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5615 mL | 12.8074 mL | 25.6148 mL | 51.2295 mL | 64.0369 mL |
| 5 mM | 0.5123 mL | 2.5615 mL | 5.123 mL | 10.2459 mL | 12.8074 mL |
| 10 mM | 0.2561 mL | 1.2807 mL | 2.5615 mL | 5.123 mL | 6.4037 mL |
| 50 mM | 0.0512 mL | 0.2561 mL | 0.5123 mL | 1.0246 mL | 1.2807 mL |
| 100 mM | 0.0256 mL | 0.1281 mL | 0.2561 mL | 0.5123 mL | 0.6404 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BAY 61-3606 is a selective and orally available inhibitor of spleen tyrosine kinase (syk) with IC50 value of 10nM [1].
The spleen tyrosine kinase plays important roles in various inflammation pathways through affecting both the FcεRI-mediated signaling in mast cells and basophils and the FcγR-mediated signaling in macrophages and neutrophils. Therefor syk is thought to be an attractive target for the treatment of related respiratory diseases such as asthma and allergy. As a potent and selective inhibitor of syk, BAY 61-3606 dose-dependently inhibited syk activity with IC50 value of 10 nM and competed against ATP with Ki value of 7.5 nM. It showed no significant effects on other tyrosine kinases including Src, Fyn, Lyn, Btk and Itk at concentration up to 4.7 μM. BAY 61-3606 inhibited the inflammation-related cell functions in various inflammatory cells. It also showed potent anti-allergic and anti-asthmatic activities in animal models [1].
In the ratbasophilic leukemia cell line, RBL-2H3 cells, treatment of BAY 61-3606 inhibited the FcεRI-mediated hexosaminidase release with IC50 value of 46 nM. In rat peritoneal mast cells, BAY 61-3606 inhibited FcεRI-mediated Serotonin release with IC50 value of 17 nM. In HCMC cells, BAY 61-3606 inhibited the release of tryptase and histamine with IC50 values of 5.5 and 5.1 nM, respectively. BAY 61-3606 also suppressed BCR-induced increases of intracellular calcium concentration with IC50 value of 81 nM. Besides that, BAY 61-3606 was reported to inhibit the FcγR-mediated superoxide production in U937 cells with IC50 value of 52 nM [1].
In rats with type-I allergic reactions, oral administration of BAY 61-3606inhibited the PCA reaction dose-dependently with ED50 value of 8 mg/kg. In an asthmatic model, administration of BAY 61-3606 at dose of 3 mg/kg significantly inhibited the increase of pulmonary pressure stimulated by DNP-BSA. In addition, administration of BAY 61-3606 at dose of 30 mg/kg resulted in the inhibition of eosinophil accumulation in BAL fluid [1].
References:
[1] Yamamoto N, Takeshita K, Shichijo M, Kokubo T, Sato M, Nakashima K, Ishimori M, Nagai H, Li YF, Yura T, Bacon KB. The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61-3606) blocks antigen-induced airway inflammation in rodents. J Pharmacol Exp Ther. 2003 Sep;306(3):1174-81.
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
- SCH 50911
Catalog No.:BCC5692
CAS No.:733717-87-8
- SB 706375
Catalog No.:BCC6256
CAS No.:733734-61-7
- AR 231453
Catalog No.:BCC5143
CAS No.:733750-99-7
- Norandrostenedione
Catalog No.:BCC9103
CAS No.:734-32-7
- Dehydrobruceine A
Catalog No.:BCN7620
CAS No.:73435-47-9
- 1-Deoxymannojirimycin hydrochloride
Catalog No.:BCC6995
CAS No.:73465-43-7
The orally available spleen tyrosine kinase inhibitor 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]nicotinamide dihydrochloride (BAY 61-3606) blocks antigen-induced airway inflammation in rodents.[Pubmed:12766258]
J Pharmacol Exp Ther. 2003 Sep;306(3):1174-81.
Spleen tyrosine kinase (Syk) tyrosine kinase plays essential roles in receptors for Fc portion of immunoglobulins and B cell receptor complex signaling in various inflammatory cells; therefore, inhibitors of Syk kinase may show potential as antiasthmatic/allergic therapeutics. We identified 2-[7-(3,4-dimethoxyphenyl)-imidazo[1,2-c]pyrimidin-5-ylamino]-nicotinamide dihydrochloride (BAY 61-3606), a potent (Ki = 7.5 nM) and selective inhibitor of Syk kinase. BAY 61-3606 inhibited not only degranulation (IC50 values between 5 and 46 nM) but also lipid mediator and cytokine synthesis in mast cells. BAY 61-3606 was highly efficacious in basophils obtained from healthy human subjects (IC50 = 10 nM) and seems to be at least as potent in basophils obtained from atopic (high serum IgE) subjects (IC50 = 8.1 nM). B cell receptor activation and receptors for Fc portion of IgG signaling in eosinophils and monocytes were also potently suppressed by BAY 61-3606. Oral administration of BAY 61-3606 to rats significantly suppressed antigen-induced passive cutaneous anaphylactic reaction, bronchoconstriction, and bronchial edema at 3 mg/kg. Furthermore, BAY 61-3606 attenuated antigen-induced airway inflammation in rats. Based on these anti-inflammatory effects of BAY 61-3606 both in vitro and in vivo, it was demonstrated that Syk may play a very critical role in the pathogenesis of allergic reactions.
Bay 61-3606 Sensitizes TRAIL-Induced Apoptosis by Downregulating Mcl-1 in Breast Cancer Cells.[Pubmed:26720004]
PLoS One. 2015 Dec 31;10(12):e0146073.
Breast cancer cells generally develop resistance to TNF-Related Apoptosis-Inducing Ligand (TRAIL) and, therefore, assistance from sensitizers is required. In our study, we have demonstrated that Spleen tyrosine kinase (Syk) inhibitor BAY 61-3606 was identified as a TRAIL sensitizer. Amplification of TRAIL-induced apoptosis by BAY 61-3606 was accompanied by the strong activation of Bak, caspases, and DNA fragmentation. In mechanism of action, BAY 61-3606 sensitized cells to TRAIL via two mechanisms regulating myeloid cell leukemia sequence-1 (Mcl-1). First, BAY 61-3606 triggered ubiquitin-dependent degradation of Mcl-1 by regulating Mcl-1 phosphorylation. Second, BAY 61-3606 downregulates Mcl-1 expression at the transcription level. In this context, BAY 61-3606 acted as an inhibitor of Cyclin-Dependent Kinase (CDK) 9 rather than Syk. In summary, BAY 61-3606 downregulates Mcl-1 expression in breast cancer cells and sensitizes cancer cells to TRAIL-mediated apoptosis.
BAY 61-3606, CDKi, and sodium butyrate treatments alter gene expression in human vestibular schwannomas and cause cell death in vitro.[Pubmed:23304241]
Ecancermedicalscience. 2012;6:285.
BACKGROUND: Disrupted kinase and signaling pathways are found in many human cancers and they are implicated in carcinogenesis. Therefore, kinases have been important targets for the development of cancer therapeutics. Human vestibular schwannomas (VS) are the third most common intracranial tumours which occur in the vestibular branch of VIII(th ) cranial nerve. Sodium butyrate (Na-Bu) is a potent histone deacetylase inhibitor (HDACi) and with therapeutic efficacy. Spleen tyrosine kinase (Syk) has been implicated in many immunological consequences and is a putative target for cancer treatment. AIMS AND OBJECTIVES: The present study was undertaken in order to evaluate the effect Na-Bu, 2,4-Diamino-5-oxo-pyrimidine hydrochloride (CDKi), a broad spectrum kinase inhibitor and BAY 61-3606 (Syk inhibitor) on the survival of VS tumour tissues in vitro and their possible effects on cell survival/death and levels of a few key proteins in the treated cells as compared to the untreated cells. MATERIALS AND METHODS: Fresh tumour tissues were collected randomly from 16 patients with sporadic, VS tumours, minced into pieces and maintained in primary cultures. Twenty four hours later these cells were exposed to Na-Bu, BAY 61-3606 or CDKi. Forty eight hours after exposure, the tissue lysates were analysed by western blotting for expression of pRb and other proteins involved in cell survival/death. SUMMARY AND SIGNIFICANCE OF THE FINDINGS: The tissue samples used were positive for S100A protein, the maker for schwann cells confirming the VS tumour samples. The three individual treatments led to morphological change, DNA fragmentation and cell death and significantly reduced level of total and phosphorylated forms of pRb protein and drastically reduced EGF-R protein. These treatments also modulated levels of other proteins involved in cell survival/death such as PI3K, Caspase 3, TGF-beta1, JNK, ASK1, Shh, NF-kappaB, p21(cip1/waf1). The Untreated cells had uncleaved PARP-1 protein and the treated cells had cleaved PARP-1. The results show that the observed cell death in treated cells perhaps is mediated by modulation of the levels and processing of certain key proteins. The possible development of these components as therapeutics is discussed.


