FlorfenicolCAS# 73231-34-2 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73231-34-2 | SDF | Download SDF |
| PubChem ID | 114811 | Appearance | Powder |
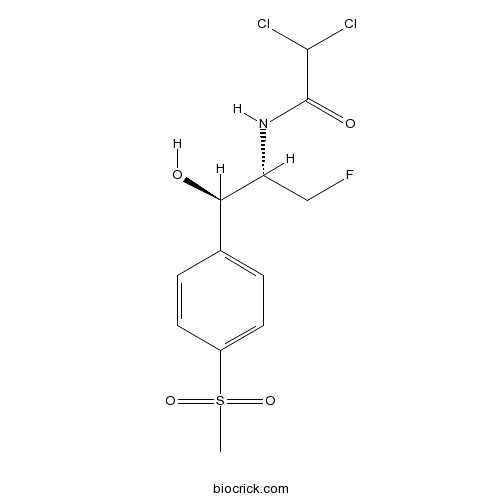
| Formula | C12H14Cl2FNO4S | M.Wt | 358 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (-)-Florfenicol; SCH-25298 | ||
| Solubility | DMSO : 100 mg/mL (279.17 mM; Need ultrasonic) | ||
| Chemical Name | 2,2-dichloro-N-[(1R,2S)-3-fluoro-1-hydroxy-1-(4-methylsulfonylphenyl)propan-2-yl]acetamide | ||
| SMILES | CS(=O)(=O)C1=CC=C(C=C1)C(C(CF)NC(=O)C(Cl)Cl)O | ||
| Standard InChIKey | AYIRNRDRBQJXIF-NXEZZACHSA-N | ||
| Standard InChI | InChI=1S/C12H14Cl2FNO4S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-15)16-12(18)11(13)14/h2-5,9-11,17H,6H2,1H3,(H,16,18)/t9-,10-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Florfenicol Dilution Calculator

Florfenicol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7933 mL | 13.9665 mL | 27.933 mL | 55.8659 mL | 69.8324 mL |
| 5 mM | 0.5587 mL | 2.7933 mL | 5.5866 mL | 11.1732 mL | 13.9665 mL |
| 10 mM | 0.2793 mL | 1.3966 mL | 2.7933 mL | 5.5866 mL | 6.9832 mL |
| 50 mM | 0.0559 mL | 0.2793 mL | 0.5587 mL | 1.1173 mL | 1.3966 mL |
| 100 mM | 0.0279 mL | 0.1397 mL | 0.2793 mL | 0.5587 mL | 0.6983 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Florfenicol, a commonly used veterinary antibiotic, is currently indicated for the treatment of bovine respiratory disease, and also used in aquaculture for the control of enteric septicemia in catfish. Florfenicol can induce early embryonic death in eggs, with an LC50 of 1.07 μg/g.
References:
[1]. Al-Shahrani S, et al. Florfenicol induces early embryonic death in eggs collected from treated hens. BMC Vet Res. 2015 Aug 18;11(1):213.
[2]. Corinna Kehrenberg, et al. A new mechanism for chloramphenicol, florfenicol and clindamycin resistance: methylation of 23S ribosomal RNA at A2503. Molecular Microbiology Volume 57, Issue 4, pages 1064–1073, August 2005
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
- 5-O-Caffeoylshikimic acid
Catalog No.:BCN7929
CAS No.:73263-62-4
- 1,4-Dihydro-1,2-dimethyl-4-oxo-3-quinolinecarboxylic acid
Catalog No.:BCN1369
CAS No.:73281-83-1
- Deoxynojirimycin hydrochloride
Catalog No.:BCN2626
CAS No.:73285-50-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
Development and Comparison of Liquid-liquid Extraction and Accelerated Solvent Extraction Methods for Quantitative Analysis of Chloramphenicol, Thiamphenicol, Florfenicol and Florfenicol Amine in Poultry Eggs.[Pubmed:30908762]
J Mass Spectrom. 2019 Mar 25.
Accelerated solvent extraction was investigated as a novel alternative technology for the separation and quantitative analysis of chloramphenicol, thiamphenicol, Florfenicol and Florfenicol amine from poultry eggs, and the results were compared with the results of liquid-liquid extraction. Rapid quantification of the target compounds was carried out by ultra-performance liquid chromatography-electrospray ionization tandem triple quadrupole mass spectrometry. This optimized method was validated according to the requirements defined by the European Union and the United States Food and Drug Administration. Finally, the new approach was successfully applied to the quantitative determination of these analytes in 90 commercial poultry eggs from local supermarkets.
Development and application of a population physiologically based pharmacokinetic model for florfenicol and its metabolite florfenicol amine in cattle.[Pubmed:30825586]
Food Chem Toxicol. 2019 Apr;126:285-294.
Florfenicol (FF) is used in cattle to treat respiratory diseases but could result in tissue residues. This study aimed to develop a population physiologically based pharmacokinetic (PBPK) model to predict the concentrations of FF and its metabolite, Florfenicol amine (FFA), in cattle after four different routes of administration, and to calculate and compare the withdrawal intervals (WDIs) with approved withdrawal times based on different marker residues and their MRLs or tolerances. A flow-limited PBPK model including both FF and FFA sub-models were developed with published data using acslXtreme. This model predicted FF and FFA concentrations in tissues and plasma/serum after intramuscular or subcutaneous administration. Based on the model, the WDIs of 46 and 58 days were calculated to ensure that total residue concentrations (FF + FFA) in 95th percentile of the population after intramuscular and subcutaneous administration were below the MRL, respectively. WDIs were calculated as 44 and 47 days to ensure that FFA concentrations after intramuscular and subcutaneous administration fell below tolerances in 99th percentile of the population, respectively. WDIs were longer than the corresponding label in China, US, and EU. This model provides a useful tool to predict tissue residues of FF and FFA in cattle to improve food safety.
Response of Freshwater Biofilms to Antibiotic Florfenicol and Ofloxacin Stress: Role of Extracellular Polymeric Substances.[Pubmed:30818877]
Int J Environ Res Public Health. 2019 Feb 27;16(5). pii: ijerph16050715.
Antibiotic residues have been detected in aquatic environments worldwide. Biofilms are one of the most successful life forms, and as a result are ubiquitous in natural waters. However, the response mechanism of freshwater biofilms to the stress of various antibiotic residues is still unclear. Here, the stress of veterinary antibiotic Florfenicol (FF) and fluoroquinolone antibiotic ofloxacin (OFL) on freshwater biofilms were investigated by determining the changes in the key physicochemical and biological properties of the biofilms. The results showed that the chlorophyll a content in biofilms firstly decreased to 46(-)71% and then recovered to original content under the stress of FF and OFL with high, mid, and low concentrations. Meanwhile, the activities of antioxidant enzymes, including superoxide dismutase and catalase, increased between 1.3(-)6.7 times their initial values. FF was more toxic to the biofilms than OFL. The distribution coefficients of FF and OFL binding in extracellular polymeric substances (EPS)-free biofilms were 3.2 and 6.5 times higher than those in intact biofilms, respectively. It indicated that EPS could inhibit the FF and OFL accumulation in biofilm cells. The present study shows that the EPS matrix, as the house of freshwater biofilms, is the primary barrier that resists the stress from antibiotic residues.
Resistant cutoff values and optimal scheme establishments for florfenicol against Escherichia coli with PK-PD modeling analysis in pigs.[Pubmed:30801741]
J Vet Pharmacol Ther. 2019 Feb 22.
Florfenicol, a structural analog of thiamphenicol, has broad-spectrum antibacterial activity against gram-negative and gram-positive bacteria. This study was conducted to investigate the epidemiological, pharmacokinetic-pharmacodynamic cutoff, and the optimal scheme of Florfenicol against Escherichia coli (E. coli) with PK-PD integrated model in the target infectious tissue. 220 E. coli strains were selected to detect the susceptibility to Florfenicol, and a virulent strain P190, whose minimum inhibitory concentration (MIC) was similar to the MIC50 (8 mug/ml), was analyzed for PD study in LB and ileum fluid. The MIC of P190 in the ileum fluid was 0.25 times lower than LB. The ratios of MBC/MIC were four both in the ileum and LB. The characteristics of time-killing curves also coincided with the MBC determination. The recommended dosages (30 mg/kg.body weight) were orally administrated in healthy pigs, and both plasma and ileum fluid were collected for PK study. The main pharmacokinetics (PK) parameters including AUC24 hr , AUC0-infinity , Tmax , T1/2 , Cmax , CLb, and Ke were 49.83, 52.33 mug*h/ml, 1.32, 10.58 hr, 9.12 mug/ml, 0.50 L/hr*kg, 0.24 hr(-1) and 134.45, 138.71 mug*hr/ml, 2.05, 13.01 hr, 16.57 mug/ml, 0.18 L/hr*kg, 0.14 hr(-1) in the serum and ileum fluid, respectively. The optimum doses for bacteriostatic, bactericidal, and elimination activities were 29.81, 34.88, and 36.52 mg/kg for 50% target and 33.95, 39.79, and 42.55 mg/kg for 90% target, respectively. The final sensitive breakpoint was defined as 16 mug/ml. The current data presented provide the optimal regimens (39.79 mg/kg) and susceptible breakpoint (16 mug/ml) for clinical use, but these predicted data should be validated in the clinical practice.


