ArecolineCAS# 73069-28-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73069-28-9 | SDF | Download SDF |
| PubChem ID | 2230 | Appearance | Powder |
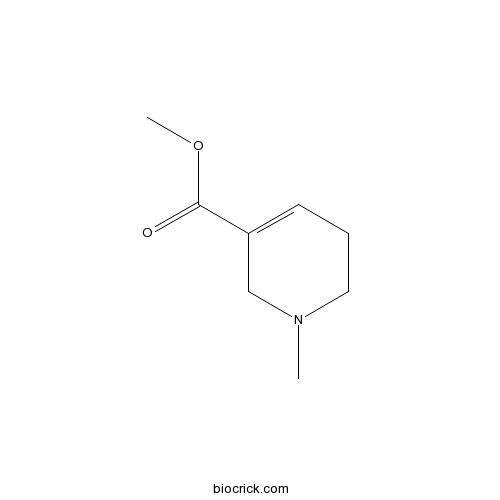
| Formula | C8H13NO2 | M.Wt | 155.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 63-75-2;Arecaline;Arecholine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | methyl 1-methyl-3,6-dihydro-2H-pyridine-5-carboxylate | ||
| SMILES | CN1CCC=C(C1)C(=O)OC | ||
| Standard InChIKey | HJJPJSXJAXAIPN-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C8H13NO2/c1-9-5-3-4-7(6-9)8(10)11-2/h4H,3,5-6H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Arecoline Dilution Calculator

Arecoline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4437 mL | 32.2186 mL | 64.4371 mL | 128.8743 mL | 161.0929 mL |
| 5 mM | 1.2887 mL | 6.4437 mL | 12.8874 mL | 25.7749 mL | 32.2186 mL |
| 10 mM | 0.6444 mL | 3.2219 mL | 6.4437 mL | 12.8874 mL | 16.1093 mL |
| 50 mM | 0.1289 mL | 0.6444 mL | 1.2887 mL | 2.5775 mL | 3.2219 mL |
| 100 mM | 0.0644 mL | 0.3222 mL | 0.6444 mL | 1.2887 mL | 1.6109 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- (+)-Praeruptorin A
Catalog No.:BCN4989
CAS No.:73069-27-9
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- Florfenicol
Catalog No.:BCC8984
CAS No.:73231-34-2
- Methylnaltrexone Bromide
Catalog No.:BCC1740
CAS No.:73232-52-7
- Moringin
Catalog No.:BCN7722
CAS No.:73255-40-0
Development and validation of a rapid LC-MS/MS method for simultaneous quantification of arecoline and its two active metabolites in rat plasma and its application to a pharmacokinetic study.[Pubmed:29573735]
J Pharm Biomed Anal. 2018 May 30;154:397-403.
Arecoline is the primary active and toxic constituent of areca nut. Arecaidine and Arecoline N-oxide are two major active metabolites of Arecoline. In this work, an accurate and simple high performance liquid chromatography tandem mass spectrometry method for simultaneous quantification of Arecoline, arecaidine and Arecoline N-oxide in rat plasma was developed and fully validated to study their pharmacokinetic behaviors in rats. After extracted from rat plasma by protein precipitation with methanol and then concentrated, the analytes were chromatographic separated on a Sepax Sapphire C18 analytical column. The mobile phase consisted of methanol and 2mM ammonium acetate buffer solution containing 0.2% (v/v) formic acid (8:92, v/v) under isocratic elution. The analytes were detected by multiple reaction monitoring (MRM) with an electrospray ionization source in the positive ion mode. The transitions of m/z 156.2-->53.2,m/z 142.2-->44.2 and m/z 172.2-->60.2 were selected for Arecoline, arecaidine and Arecoline N-oxide, respectively. The method was linear over the concentration range of 0.5-100ng/mL for Arecoline, 5-5000ng/mL for arecaidine and Arecoline N-oxide with no carry-over effect. The accuracies and intra- and inter-batch precisions were all within the acceptance limits. No matrix effect and potential interconversion between the analytes and other metabolites were observed in this method. The validated method was further employed to a preclinical pharmacokinetic study of Arecoline, arecaidine and Arecoline N-oxide after oral treatment with 20mg/kg Arecoline to rats.
Hinokitiol ablates myofibroblast activation in precancerous oral submucous fibrosis by targeting Snail.[Pubmed:29328529]
Environ Toxicol. 2018 Apr;33(4):454-462.
Oral submucous fibrosis (OSF) is a precancerous condition with symptoms of limited mouth opening and areca nut chewing habit has been implicated in its pathogenesis. Hinokitiol, a natural tropolone derived from Chamacyparis taiwanensis, has been reported to improve oral lichen planus and inhibit various cancer cells. Here, we showed that hinokitiol reduced the myofibroblast activities in fBMFs and prevented the Arecoline-induced transdifferentiation. Treatment of hinokitiol dose-dependently downregulated the myofibroblast markers as well as various EMT transcriptional factors. In particular, we identified that Snail was able to bind to the E-box in the alpha-SMA promoter. Our data suggested that exposure of fBMFs to hinokitiol mitigated the hallmarks of myofibroblasts, while overexpression of Snail eliminated the effect of hinokitiol. These findings revealed that the inhibitory effect of hinokitiol on myofibroblasts was mediated by repression of alpha-SMA via regulation of Snail and showed the anti-fibrotic potential of hinokitiol in the treatment of OSF.
Cytotoxic, embryotoxic, insecticidal and anti-microbial activities of standardized Areca catechu nut.[Pubmed:29618425]
Pak J Pharm Sci. 2018 Mar;31(2):385-392.
The study was aimed at evaluating various biological actions of widely consumed Areca catechu nut. The nut's ethanolic extract exhibited cytotoxicity (lung cancer cell line), embryotoxicity (chick embryo), phytotoxicity (Lemna minor), insecticidal (Rhyzopertha dominica), anti-bacterial (Pseudomonas aeruginosa), anti-fungal (Microsporum canis) and mitogenic (human blood lymphocytes) actions. The standardization results revealed presence of 1.7 mu g Arecoline per mg of extract. In conclusion, the Areca nut is endowed with both harmful and beneficial biological actions. Keeping in view its wide consumption and ease of availability, the aforesaid information should be channelized for health and agricultural benefits.
Arecoline ameliorates hyperthyroid condition in mice under cold stress.[Pubmed:29278926]
Arch Physiol Biochem. 2018 Dec;124(5):436-441.
Betel nut of Areca catechu is chewed by millions of people for increased capacity to work and stress reduction, but it contains Arecoline that causes hypothyroidism. The aim is to investigate the role of Arecoline on thyroid activity in cold stress in mice. Arecoline treatment (10 mg/kg body wt/day, for 7 d) caused a reduction in thyroid weight and ultrastructural degeneration of thyro-follicular cells with depletion of T3 and T4 levels compared with the control mice. Cold stress (4 degrees C for 2 h, twice daily, for 7 d) stimulated thyroid activity ultrastructurally with an elevation of T3 and T4 levels. Arecoline treatment in cold stress suppressed thyroid activity by showing reversed changes to those of cold stress. In contrast, TSH concentrations were consistently increased under all experimental conditions. The findings suggest that cold stress causes hyperthyroidism which Arecoline can ameliorate in mice.
Butylidenephthalide abrogates the myofibroblasts activation and mesenchymal transdifferentiation in oral submucous fibrosis.[Pubmed:29665273]
Environ Toxicol. 2018 Jun;33(6):686-694.
Oral submucous fibrosis (OSF) is a premalignant disorder in the oral cavity, and areca nut chewing habit has been implicated in the persistent activation of myofibroblasts and the subsequent fibrosis. Therefore, it is critical to ameliorate the excessive activities of myofibroblasts prior to the malignant transformation of OSF. In the current study, we evaluated the cytotoxicity of butylidenephthalide (BP), a major phthalide ingredient of Angelica sinensis, in fibrotic buccal mucosal fibroblasts (fBMFs) as well as various myofibroblast hallmarks, including the phenotypical characteristics and fibrosis-related markers. Our results demonstrated that myofibroblast activities, including collagen gel contraction, migration, invasion and wound healing abilities were inhibited in response to BP. The expression levels of myofibroblast marker, alpha-smooth muscle actin (alpha-SMA), fibronectin and type 1 collagen A1 were decreased after exposure of BP. Moreover, we found that the EMT-related markers, Twist, Snail and ZEB1 were all downregulated after BP treatment. Most importantly, our findings demonstrated that BP impeded the binding of Snail to the E-box region in the alpha-SMA promoter, which may lead to inhibition of the Arecoline-induced myofibroblast activities. Collectively, our data indicated that BP reduced numerous myofibroblast features in fBMFs and hindered the binding of Snail to alpha-SMA, thereby may function as an effective and natural antifibrosis compound.
Effects of arecoline on proliferation of oral squamous cell carcinoma cells by dysregulating c-Myc and miR-22, directly targeting oncostatin M.[Pubmed:29385191]
PLoS One. 2018 Jan 31;13(1):e0192009.
Arecoline, the major alkaloid of areca nut, is known to induce oral carcinogenesis, however, its mechanism is still needed to elucidate. This study investigated the effects of Arecoline on cell viability and cell-cycle progression of oral squamous cell carcinoma (OSCC) cells as well as a relevant cellular gene expression. The results showed that a low concentration of Arecoline (0.025 mug/ml) increased OSCC cell viability, proportion of cells in G2/M phase and cell proliferation. Simultaneously, it induced IL-6, STAT3 and c-Myc expression. Interestingly, c-myc promoter activity was also induced by Arecoline. MiR-22 expression in Arecoline-treated OSCC cells was suppressed and comparable to an upregulated c-Myc expression. In Arecoline-treated OSCC cells, oncostatin M (OSM) expression was significantly upregulated and inversely correlated with miR-22 expression. Likewise, OSM expression and its post-transcriptional activity were significantly decreased in miR-22-transfected OSCC and 293FT cells. This result demonstrated that miR-22 directly targeted OSM. Interestingly, miR-22 played an important role as a tumor suppresser on suppressing cell proliferation, migration and cell-cycle progression of OSCC cells. This result suggested the effect of Arecoline to promote cell proliferation and cell-cycle progression of OSCC cells might be involved in induction of c-Myc expression and reduction of miR-22 resulting in OSM upregulation.


