(+)-Praeruptorin ACAS# 73069-27-9 |
- (-)-Praeruptorin A
Catalog No.:BCN7664
CAS No.:14017-71-1
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73069-27-9 | SDF | Download SDF |
| PubChem ID | 38347607 | Appearance | Powder |
| Formula | C21H22O7 | M.Wt | 386.4 |
| Type of Compound | Coumarins | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
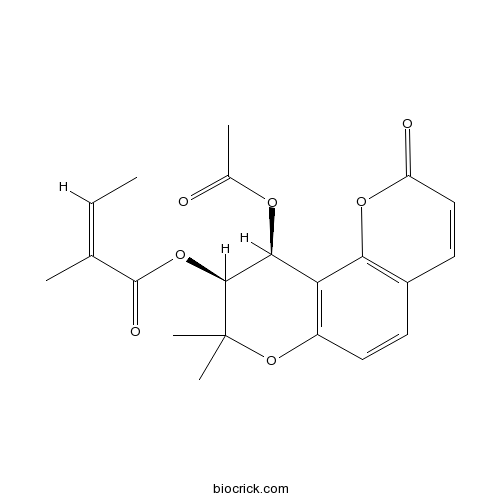
| Chemical Name | [(9S,10S)-10-acetyloxy-8,8-dimethyl-2-oxo-9,10-dihydropyrano[2,3-f]chromen-9-yl] (Z)-2-methylbut-2-enoate | ||
| SMILES | CC=C(C)C(=O)OC1C(C2=C(C=CC3=C2OC(=O)C=C3)OC1(C)C)OC(=O)C | ||
| Standard InChIKey | XGPBRZDOJDLKOT-NXIDYTHLSA-N | ||
| Standard InChI | InChI=1S/C21H22O7/c1-6-11(2)20(24)27-19-18(25-12(3)22)16-14(28-21(19,4)5)9-7-13-8-10-15(23)26-17(13)16/h6-10,18-19H,1-5H3/b11-6-/t18-,19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (+)-Praeruptorin A exerts distinct relaxant effects on isolated rat aorta rings, which may be mainly attributed to nitric oxide synthesis catalyzed by endothelial nitric oxide synthase. |
| Targets | NOS | Calcium Channel |
| In vitro | (+/-)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis.[Pubmed: 20433815]Chem Biol Interact. 2010 Jul 30;186(2):239-46.
|

(+)-Praeruptorin A Dilution Calculator

(+)-Praeruptorin A Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.588 mL | 12.94 mL | 25.8799 mL | 51.7598 mL | 64.6998 mL |
| 5 mM | 0.5176 mL | 2.588 mL | 5.176 mL | 10.352 mL | 12.94 mL |
| 10 mM | 0.2588 mL | 1.294 mL | 2.588 mL | 5.176 mL | 6.47 mL |
| 50 mM | 0.0518 mL | 0.2588 mL | 0.5176 mL | 1.0352 mL | 1.294 mL |
| 100 mM | 0.0259 mL | 0.1294 mL | 0.2588 mL | 0.5176 mL | 0.647 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Praeruptorin A
Catalog No.:BCN4987
CAS No.:73069-25-7
- Atractylenolide II
Catalog No.:BCN1044
CAS No.:73069-14-4
- Atractylenolide I
Catalog No.:BCN1043
CAS No.:73069-13-3
- Epigomisin O
Catalog No.:BCN2862
CAS No.:73036-31-4
- Atractylenolide III
Catalog No.:BCN1045
CAS No.:73030-71-4
- CyPPA
Catalog No.:BCC7526
CAS No.:73029-73-9
- 15-Isopimarene-8,18-diol
Catalog No.:BCN4287
CAS No.:73002-86-5
- Lidocaine hydrochloride
Catalog No.:BCC9009
CAS No.:73-78-9
- Guanine
Catalog No.:BCN8414
CAS No.:73-40-5
- H-Ile-OH
Catalog No.:BCC2960
CAS No.:73-32-5
- Melatonin
Catalog No.:BCN2196
CAS No.:73-31-4
- Adenine
Catalog No.:BCC4450
CAS No.:73-24-5
- Praeruptorin D
Catalog No.:BCN4990
CAS No.:73069-28-0
- Scutebarbatine D
Catalog No.:BCN8536
CAS No.:910099-76-2
- Arecoline
Catalog No.:BCN8537
CAS No.:73069-28-9
- Ferruginine
Catalog No.:BCN1911
CAS No.:73069-63-3
- Fenticonazole nitrate
Catalog No.:BCC8983
CAS No.:73151-29-8
- Effusol
Catalog No.:BCN2928
CAS No.:73166-28-6
- (d(CH2)51,Tyr(Me)2,Arg8)-Vasopressin
Catalog No.:BCC6011
CAS No.:73168-24-8
- Baptifoline
Catalog No.:BCN7988
CAS No.:732-50-3
- Xamoterol hemifumarate
Catalog No.:BCC6861
CAS No.:73210-73-8
- Chloranthalactone E
Catalog No.:BCN7466
CAS No.:73215-92-6
- Remoxipride hydrochloride
Catalog No.:BCC6844
CAS No.:73220-03-8
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
(+/-)-Praeruptorin A enantiomers exert distinct relaxant effects on isolated rat aorta rings dependent on endothelium and nitric oxide synthesis.[Pubmed:20433815]
Chem Biol Interact. 2010 Jul 30;186(2):239-46.
Praeruptorin A is a coumarin compound naturally occurring in the roots of Peucedanum praeruptorum Dunn., a commonly used traditional Chinese medicine for the treatment of certain respiratory diseases and hypertension. Although previous studies indicated the relaxant effects of (+/-)-praeruptorin A on tracheal and arterial preparations, little is known about the functional characteristics of the enantiomers. In the present study, the two enantiomers were successfully isolated and identified by using a preparative Daicel Chiralpak AD-H column, and their relaxant effects on aorta rings were observed and compared. (+)-Praeruptorin A showed more potent relaxation than (-)-praeruptorin A against KCl- and phenylephrine-induced contraction of rat isolated aortic rings with intact endothelium. Removal of the endothelium remarkably reduced the relaxant effect of (+)-Praeruptorin A but not that of (-)-praeruptorin A. Pretreatment of aortic rings with N(omega)-nitro-L-arginine methyl ester (L-NAME, an inhibitor of nitric oxide synthase) or methylene blue (MB, a soluble guanylyl cyclase inhibitor) resulted in similar changes of the relaxant effects of the two enantiomers to endothelium removal. Molecular docking studies also demonstrated that (+)-Praeruptorin A was in more agreement to nitric oxide synthase pharmacophores than (-)-praeruptorin A. On the other hand, the two enantiomers of praeruptorin A could slightly attenuate the contraction of rat aortic rings induced by internal Ca(2+) release from sarcoplasmic reticulum (SR). These findings indicated that (+)-Praeruptorin A and (-)-praeruptorin A exerted distinct relaxant effects on isolated rat aorta rings, which might be mainly attributed to nitric oxide synthesis catalyzed by endothelial nitric oxide synthase.


