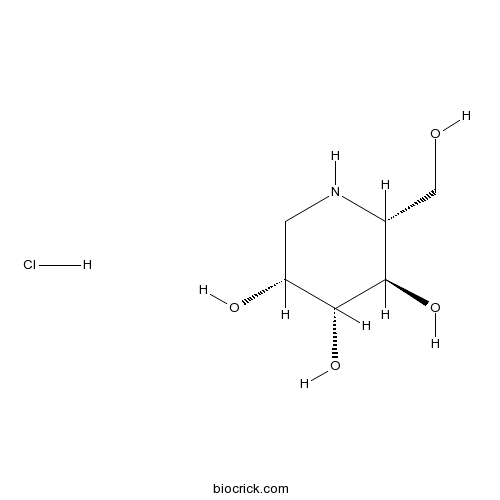
1-Deoxymannojirimycin hydrochlorideCAS# 73465-43-7 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 73465-43-7 | SDF | Download SDF |
| PubChem ID | 11390018 | Appearance | Powder |
| Formula | C6H14ClNO4 | M.Wt | 199.63 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in water | ||
| Chemical Name | (2R,3R,4R,5R)-2-(hydroxymethyl)piperidine-3,4,5-triol;hydrochloride | ||
| SMILES | C1C(C(C(C(N1)CO)O)O)O.Cl | ||
| Standard InChIKey | ZJIHMALTJRDNQI-MVNLRXSJSA-N | ||
| Standard InChI | InChI=1S/C6H13NO4.ClH/c8-2-3-5(10)6(11)4(9)1-7-3;/h3-11H,1-2H2;1H/t3-,4-,5-,6-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of α-mannosidase I. |

1-Deoxymannojirimycin hydrochloride Dilution Calculator

1-Deoxymannojirimycin hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.0093 mL | 25.0463 mL | 50.0927 mL | 100.1853 mL | 125.2317 mL |
| 5 mM | 1.0019 mL | 5.0093 mL | 10.0185 mL | 20.0371 mL | 25.0463 mL |
| 10 mM | 0.5009 mL | 2.5046 mL | 5.0093 mL | 10.0185 mL | 12.5232 mL |
| 50 mM | 0.1002 mL | 0.5009 mL | 1.0019 mL | 2.0037 mL | 2.5046 mL |
| 100 mM | 0.0501 mL | 0.2505 mL | 0.5009 mL | 1.0019 mL | 1.2523 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dehydrobruceine A
Catalog No.:BCN7620
CAS No.:73435-47-9
- Norandrostenedione
Catalog No.:BCC9103
CAS No.:734-32-7
- AR 231453
Catalog No.:BCC5143
CAS No.:733750-99-7
- SB 706375
Catalog No.:BCC6256
CAS No.:733734-61-7
- SCH 50911
Catalog No.:BCC5692
CAS No.:733717-87-8
- Niclosamide monohydrate
Catalog No.:BCC5212
CAS No.:73360-56-2
- 9-O-Acetyl-4,4'-di-O-methyllariciresinol
Catalog No.:BCN1367
CAS No.:73354-15-1
- Albanin A
Catalog No.:BCN3290
CAS No.:73343-42-7
- Macbecin I
Catalog No.:BCC7551
CAS No.:73341-72-7
- 3-Hydroxy-9,10-Dimethoxypterocarpan
Catalog No.:BCC8101
CAS No.:73340-41-7
- Methylnissolin
Catalog No.:BCN1368
CAS No.:733-40-4
- BAY 61-3606
Catalog No.:BCC1406
CAS No.:732983-37-8
- Tetrachyrin
Catalog No.:BCN4776
CAS No.:73483-88-2
- 7-Acetyllycopsamine
Catalog No.:BCN2000
CAS No.:73544-48-6
- cis-ACBD
Catalog No.:BCC6587
CAS No.:73550-55-7
- Mevastatin
Catalog No.:BCN2568
CAS No.:73573-88-3
- 27-p-Coumaroyloxyursolic acid
Catalog No.:BCN4288
CAS No.:73584-67-5
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
- (-)-Bicuculline methobromide
Catalog No.:BCC6555
CAS No.:73604-30-5
- 3-Hydroxybenzylamine
Catalog No.:BCN1804
CAS No.:73604-31-6
- Xylazine
Catalog No.:BCC5167
CAS No.:7361-61-7
- 1-(4-Methoxycinnamoyl)pyrrole
Catalog No.:BCN4027
CAS No.:736140-70-8
- 5-Amino-1-(2-hydroxyethyl)pyrazole
Catalog No.:BCC8727
CAS No.:73616-27-0
- p-Coumaric acid ethyl ester
Catalog No.:BCN4289
CAS No.:7362-39-2
Hiding inside? Intracellular expression of non-glycosylated c-kit protein in cardiac progenitor cells.[Pubmed:27161312]
Stem Cell Res. 2016 May;16(3):795-806.
Cardiac progenitor cells including c-kit(+) cells and cardiosphere-derived cells (CDCs) play important roles in cardiac repair and regeneration. CDCs were reported to contain only small subpopulations of c-kit(+) cells and recent publications suggested that depletion of the c-kit(+) subpopulation of cells has no effect on regenerative properties of CDCs. However, our current study showed that the vast majority of CDCs from murine heart actually express c-kit, albeit, in an intracellular and non-glycosylated form. Immunostaining and flow cytometry showed that the fluorescent signal indicative of c-kit immunostaining significantly increased when cell membranes were permeabilized. Western blots further demonstrated that glycosylation of c-kit was increased during endothelial differentiation in a time dependent manner. Glycosylation inhibition by 1-Deoxymannojirimycin hydrochloride (1-DMM) blocked c-kit glycosylation and reduced expression of endothelial cell markers such as Flk-1 and CD31 during differentiation. Pretreatment of these cells with a c-kit kinase inhibitor (imatinib mesylate) also attenuated Flk-1 and CD31 expression. These results suggest that c-kit glycosylation and its kinase activity are likely needed for these cells to differentiate into an endothelial lineage. In vivo, we found that intracellular c-kit expressing cells are located in the wall of cardiac blood vessels in mice subjected to myocardial infarction. In summary, our work demonstrated for the first time that c-kit is not only expressed in CDCs but may also directly participate in CDC differentiation into an endothelial lineage.
The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells.[Pubmed:2937779]
J Biol Chem. 1986 Apr 5;261(10):4766-74.
The mannose analogue, 1-deoxymannojirimycin, which inhibits Golgi alpha-mannosidase I but not endoplasmic reticulum (ER) alpha-mannosidase has been used to determine the role of the ER alpha-mannosidase in the processing of the asparagine-linked oligosaccharides on glycoproteins in intact cells. In the absence of the inhibitor, the predominant oligosaccharide structures found on the ER glycoprotein 3-hydroxy-3-methylglutaryl-CoA reductase in UT-1 cells are single isomers of Man6GlcNAc and Man8GlcNAc. In the presence of 150 microM 1-deoxymannojirimycin, the Man8GlcNAc2 isomer accumulates indicating that the 1-deoxymannojirimycin-resistant ER alpha-mannosidase is responsible for the conversion of Man9GlcNAc2 to Man8GlcNAc2 on reductase. The processing of Man8GlcNAc2 to Man6GlcNAc2, however, must be attributed to a 1-deoxymannojirimycin-sensitive alpha-mannosidase. When cells were radiolabeled with [2-(3)H]mannose for 15 h in the presence of 1-deoxymannojirimycin and then further incubated for 3 h in nonradioactive medium without inhibitor, the Man8GlcNAc2 oligosaccharides which accumulated during the labeling period were partially trimmed to Man6GlcNAc. This finding suggests that a second alpha-mannosidase, sensitive to 1-deoxymannojirimycin, resides in the crystalloid ER and is responsible for trimming the reductase oligosaccharide chain from Man8GlcNAc2 to Man6GlcNAc2. To determine if ER alpha-mannosidase is responsible for trimming the oligosaccharides of all glycoproteins from Man9GlcNAc to Man8GlcNAc, the total asparagine-linked oligosaccharides of rat hepatocytes labeled with [2-(3)H]mannose in the presence or absence of 1.0 mM 1-deoxymannojirimycin were examined. the inhibitor prevented the formation of complex oligosaccharides and caused a 30-fold increase in the amount of Man9GlcNAc2 and a 13-fold increase in the amount of Man8GlcNAc2 present on secreted glycoproteins. This result suggests that only one-third of the secreted glycoproteins is initially processed by ER alpha-mannosidase, and two-thirds are processed by Golgi alpha-mannosidase I or another 1-deoxymannojirimycin-sensitive alpha-mannosidase. The inhibitor caused only a 2.6-fold increase in the amount of Man9GlcNAc2 on cellular glycoproteins suggesting that a higher proportion of these glycoproteins are initially processed by the ER alpha-mannosidase. We conclude that some, but not all, hepatocyte glycoproteins are substrates for ER alpha-mannosidase which catalyzes the removal of a specific mannose residue from Man9GlcNAc2 to form a single isomer of Man8GlcNAc2.
The effect of 1-deoxymannojirimycin on rat liver alpha-mannosidases.[Pubmed:6239623]
Biochem Biophys Res Commun. 1984 Nov 30;125(1):324-31.
The mannose analogue, 1-deoxymannojirimycin, has been tested for its effect on five alpha-mannosidase activities present in rat liver and shown to be a specific inhibitor of Golgi alpha-mannosidase I at low mumolar concentrations. Golgi alpha-mannosidases I and II were assayed in a highly purified Golgi membrane preparation. Endoplasmic reticulum alpha-mannosidase activity was measured in a rough endoplasmic reticulum detergent extract. A purified soluble alpha-mannosidase activity which we believe is derived from the endoplasmic reticulum during tissue homogenization was also tested. And finally, the lysosomal or acidic alpha-mannosidase was measured in a postnuclear supernatant fraction obtained from rat liver. The results presented here show that 1-deoxymannojirimycin inhibits only Golgi alpha-mannosidase I, which is consistent with its effect on oligosaccharide processing in vivo (Fuhrmann et al. Nature 1984 307:755-758).
Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides.[Pubmed:6230538]
Nature. 1984 Feb 23-29;307(5953):755-8.
Many secretory and membrane proteins are glycoproteins carrying asparagine-linked (N-linked) oligosaccharides. There are two types of N-linked glycans, referred to as high-mannose and complex type, respectively. Biosynthesis of N-linked glycans of the complex type proceeds via a high-mannose intermediate. After the initial transfer of a high-mannose oligosaccharide with the composition (Glc)3(Man)9(GlcNAc)2 from a lipid carrier to the nascent polypeptide chain, trimming reactions take place. Trimming glucosidases remove the glucose residues quantitatively and mannosidases IA/B and II can remove all but three mannose residues. After trimming, terminal sugars such as N-acetylglucosamine, galactose, sialic acid and fucose may be added and result in the conversion to a glycan of the complex type. Because suitable inhibitors were lacking, it was difficult to assess the importance of the trimming reactions for proper intracellular traffic, modification reactions other than the addition of terminal sugars, or as regulatory steps in glycoprotein processing. Here we describe the action of 1-deoxymannojirimycin (1,5-dideoxy-1,5-imino-D-mannitol, dMM; Fig. 1) on the biosynthesis of IgM and IgD. dMM is the mannose analogue of 1-deoxynojirimycin (dNM; Fig. 1), itself a glucosidase inhibitor. We present evidence that dMM is a mannosidase inhibitor. In vivo dMM inhibits the equivalent of the mannosidase IA/B activities and blocks conversion of high-mannose to complex oligosaccharides. It is the first such inhibitor to be reported. Interference with the biosynthetic pathway of N-linked glycans could prove to be a powerful way to manipulate carbohydrate structure in vivo.


