Ruxolitinib (INCB018424)JAK inhibitor CAS# 941678-49-5 |
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- BMS-911543
Catalog No.:BCC2204
CAS No.:1271022-90-2
- AZ 960
Catalog No.:BCC2197
CAS No.:905586-69-8
- TG101348 (SAR302503)
Catalog No.:BCC2190
CAS No.:936091-26-8
- XL019
Catalog No.:BCC2057
CAS No.:945755-56-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 941678-49-5 | SDF | Download SDF |
| PubChem ID | 25126798 | Appearance | Powder |
| Formula | C17H18N6 | M.Wt | 306.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | INCB018424 | ||
| Solubility | Soluble in DMSO > 10 mM | ||
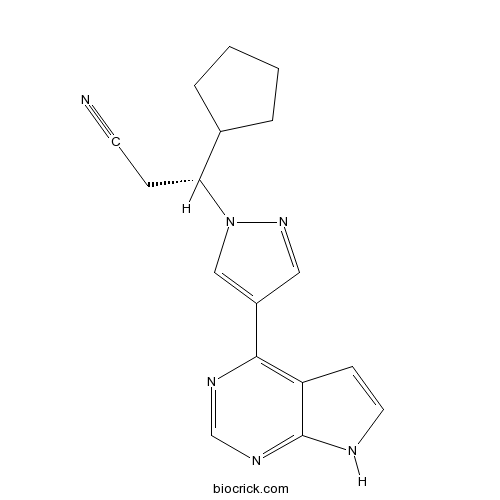
| Chemical Name | (3R)-3-cyclopentyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]propanenitrile | ||
| SMILES | C1CCC(C1)C(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3 | ||
| Standard InChIKey | HFNKQEVNSGCOJV-OAHLLOKOSA-N | ||
| Standard InChI | InChI=1S/C17H18N6/c18-7-5-15(12-3-1-2-4-12)23-10-13(9-22-23)16-14-6-8-19-17(14)21-11-20-16/h6,8-12,15H,1-5H2,(H,19,20,21)/t15-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | INCB018424 is the first potent, selective inhibitor of JAK1/2 to enter the clinic with IC50 of 3.3 nM/2.8 nM, >130-fold selectivity for JAK1/2 versus JAK3. | |||||
| Targets | JAK1 | JAK2 | ||||
| IC50 | 3.3 nM | 2.8 nM | ||||
| Cell experiment: [1] | |
| Cell lines | Primary mononuclear cells isolated from patients with PV or normal control persons |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | IC50: erythroid progenitors: 407 nM for normal donors, 223 nM for PV donors myeloid progenitors: 511 nM for normal donors, 444 nM for PV donors 14 days |
| Applications | Growth of clonogenic progenitors of erythroid (BFU-E) and myeloid origin (CFU-M) was assessed in colony-forming assays in the presence of increasing concentrations of INCB018424. Dose-dependent inhibition of the growth of erythroid and myeloid progenitors was observed with INCB018424. The mean IC50 for INCB018424 against erythroid progenitors was 407 nM for normal donors and 223 nM for PV donors. A similar effect was observed on myeloid progenitors (CFU-M), with IC50 values of 511 nM and 444 nM for control and PV samples, respectively. |
| Animal experiment: [2] | |
| Animal models | C57BL/6N mice |
| Dosage form | Oral administration, 75 mg/kg |
| Application | Mice receiving 75 mg/kg ruxolitinib or vehicle 6 hours prior to and 6 hours after injection of OVA/CpG were analyzed for expression of activation markers on CD11c 1CD81 splenic DCs. Lower expression levels of CD40, CD80, CD86 as well as MHC I and II molecules were detected in ruxolitinib-challenged animals. Next, ruxolitinib or vehicle was fed to mice 6 hours prior to as well as 6 hours and 18 hours after priming with OVA/CpG and adoptive transfer of CFSE-labeled OT-I cells. Analysis of transferred CFSE-labeled OT-I T cells revealed reduced proliferation, CD25 expression, and IFN-production in mice pretreated with ruxolitinib. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood, 2010, 115(15): 3109-3117. [2] Heine A, Held S A E, Daecke S N, et al. The JAK-inhibitor ruxolitinib impairs dendritic cell function in vitro and in vivo. Blood, 2013, 122(7): 1192-1202. | |

Ruxolitinib (INCB018424) Dilution Calculator

Ruxolitinib (INCB018424) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.264 mL | 16.3201 mL | 32.6403 mL | 65.2805 mL | 81.6007 mL |
| 5 mM | 0.6528 mL | 3.264 mL | 6.5281 mL | 13.0561 mL | 16.3201 mL |
| 10 mM | 0.3264 mL | 1.632 mL | 3.264 mL | 6.5281 mL | 8.1601 mL |
| 50 mM | 0.0653 mL | 0.3264 mL | 0.6528 mL | 1.3056 mL | 1.632 mL |
| 100 mM | 0.0326 mL | 0.1632 mL | 0.3264 mL | 0.6528 mL | 0.816 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
Ruxolitinib is a JAK1/2 inhibitor that is primarily metablized by CYP3A4. A 50% reduce in ruxolitinib dosage is suggested for the combination therapy of ruxolitinib with a potent CYP3A4 inhibitor due to greatly increased PD activity of ruxolitinib; while no ruxolitinib dosage adjustment is suggested for the combination of reuxolitinib with inducers or mild/moderate inhibitors of CYP3A4 due to no significant change in PD activity.
Abstract
The PK of ruxolitinib, a JAK1/2 inhibitor approved for the treatment of myelofibrosis, was not significantly affected by gender and body weight indicating no need to adjust ruxolitinib dose based on these two factors.
Abstract
Ruxolitinib is a JAK inhibitor that has been approved by FDA to treat advanced myelofibrosis. Since it’s a highly selective inhibitor of JAK/STAT signaling pathway, ruxolitinib significantly reduced proliferation, colony formation and expression of pSTAT1 and pSTAT3 in HCC cells.
Abstract
Ruxolitinib, a JAK1/2 inhibitor, was well tolerated in MF patients participating in a randomized trial, where ruxolitinib sustainably reduced splenomegaly in patients and prolonged overall survival of patients with manageable side effects, including anemia and thrombocytopenia.
Abstract
In the phase 3 COMFORT-II study, ruxolitinib, a JAK1/2 inhibitor, exhibited better efficacy for the treatment of MF patients than BAT in terms of HRQoL improvement, symptom relief and a few other factors.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ruxolitinib dihydrochloride is a specific inhibitor of Janus-associated kinase (JAK1 and JAK2). Ruxolitinib is a small molecular with the formula of C17H21N6O4Pand Molecular Weight of 404. Ruxolitinib phosphate is an administered ATP-competitive cyclopentylpropionitrile derivative and shows inhibition activity on JAK1 and JAK2. Ruxolitinib inhibits phosphorylation of JAK1/2, STAT5, and ERK1/2, resulting in reduced cellular proliferation.
References
1. Ruxolitinib inhibits transforming JAK2 fusion proteins in vitro and induces complete cytogenetic remission in t (8; 9)(p22; p24)/PCM1-JAK2–positive chronic. E Lierman, D Selleslag, S Smits, J Billiet. Blood. 2012
2. Ruxolitinib for the treatment of myelofibrosis. A Ostojic, R Vrhovac, S Verstovsek. Ther Clin Risk Manag. 2011
- Selaginellin
Catalog No.:BCN8215
CAS No.:941269-84-7
- 1,3,5-Cadinatriene-3,8-diol
Catalog No.:BCN4486
CAS No.:941227-27-6
- 1'-Acetonaphthone
Catalog No.:BCC8446
CAS No.:941-98-0
- KD 5170
Catalog No.:BCC2420
CAS No.:940943-37-3
- SB743921
Catalog No.:BCC4559
CAS No.:940929-33-9
- R-7128
Catalog No.:BCC1880
CAS No.:940908-79-2
- Poliumoside
Catalog No.:BCN1204
CAS No.:94079-81-9
- Suplatast Tosylate
Catalog No.:BCC4961
CAS No.:94055-76-2
- NVP-BHG712
Catalog No.:BCC3963
CAS No.:940310-85-0
- N,N'-Bis(salicylidene)ethylenediamine
Catalog No.:BCC9064
CAS No.:94-93-9
- Piperine
Catalog No.:BCN1018
CAS No.:94-62-2
- 2-Amino-6-ethoxybenzothiazole
Catalog No.:BCC8541
CAS No.:94-45-1
- INCB032304
Catalog No.:BCC6455
CAS No.:941685-27-4
- S-Ruxolitinib (INCB018424)
Catalog No.:BCC2201
CAS No.:941685-37-6
- 8beta-(4'-Hydroxytigloyloxy)costunolide
Catalog No.:BCN7885
CAS No.:94190-32-6
- Methyl 3-indolecarboxylate
Catalog No.:BCN4487
CAS No.:942-24-5
- VTP-27999
Catalog No.:BCC2048
CAS No.:942142-51-0
- SCH772984
Catalog No.:BCC1935
CAS No.:942183-80-4
- PI 828
Catalog No.:BCC7494
CAS No.:942289-87-4
- 20-Dehydroeupatoriopicrin semiacetal
Catalog No.:BCN7370
CAS No.:94234-24-9
- Scutebarbatine I
Catalog No.:BCN1026
CAS No.:960302-84-5
- Cycloart-24-ene-1alpha,2alpha,3beta-triol
Catalog No.:BCN7983
CAS No.:942407-97-8
- VKGILS-NH2
Catalog No.:BCC3953
CAS No.:942413-05-0
- Nemoralisin
Catalog No.:BCN4488
CAS No.:942480-13-9
The effect of CYP3A4 inhibition or induction on the pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in healthy volunteers.[Pubmed:21602517]
J Clin Pharmacol. 2012 Jun;52(6):809-18.
Ruxolitinib, a selective Janus kinase (JAK) 1&2 inhibitor in development for the treatment of myeloproliferative neoplasms, is primarily metabolized by CYP3A4. The effects of inhibition or induction of CYP3A4 on single oral dose ruxolitinib pharmacokinetics (PK) and pharmacodynamics (PD) were evaluated in healthy volunteers. Coadministration of ketoconazole (a potent CYP3A4 inhibitor) and erythromycin (a moderate CYP3A4 inhibitor) increased total ruxolitinib plasma exposure (AUC(0-infinity)) by 91% and 27%, respectively, and ruxolitinib PD, as measured by the inhibition of interleukin (IL)-6-stimulated STAT3 phosphorylation in whole blood, was generally consistent with the PK observed. Pretreatment with rifampin, a potent CYP3A4 inducer, decreased ruxolitinib AUC(0-infinity) by 71% while resulting in only a 10% decrease in the overall PD activity. This apparent PK/PD discrepancy may be explained, in part, by an increase in the relative abundance of ruxolitinib active metabolites with the rifampin coadministration. The collective PK/PD data suggest that starting doses of ruxolitinib should be reduced by 50% if coadministered with a potent CYP3A4 inhibitor, whereas adjustments in ruxolitinib starting doses may not be needed when coadministered with inducers or mild/moderate inhibitors of CYP3A4. All study doses of ruxolitinib were generally safe and well tolerated when given alone and in combination with ketoconazole, erythromycin, or rifampin.
Population pharmacokinetic analysis of orally-administered ruxolitinib (INCB018424 Phosphate) in patients with primary myelofibrosis (PMF), post-polycythemia vera myelofibrosis (PPV-MF) or post-essential thrombocythemia myelofibrosis (PET MF).[Pubmed:23677817]
J Clin Pharmacol. 2013 Jul;53(7):721-30.
Ruxolitinib is a selective inhibitor of Janus kinase 1 and 2, which is approved to treat intermediate or high-risk myelofibrosis. The population pharmacokinetics for ruxolitinib were characterized by a modeling dataset of 272 subjects from a Phase 2 and a Phase 3 study and validated by an external validation dataset of 142 subjects from a second Phase 3 study. The PK of ruxolitinib was adequately described by a two-compartment disposition model with first-order absorption and linear elimination. All model parameters were estimated with good precision. Gender and body weight were identified as covariates for oral clearance (CL/F) and volume of distribution for central compartment (Vc/F), respectively. Apparent oral clearance was 22.1 and 17.7 L/h for a typical male and female subject, respectively, with 39.1% unexplained inter-individual variability (IIV). The typical Vc /F for a subject with a median weight of 72.9 kg was estimated to be 58.6 L, with 28% unexplained IIV. The model predictive performance was validated by visual predictive check (VPC) and the external validation dataset. This analysis suggests that effects of gender and body weight on ruxolitinib PK are not clinically significant and hence no dose adjustment is needed based on gender and weight.
Pharmacokinetics and pharmacodynamics of orally administered ruxolitinib (INCB018424 phosphate) in renal and hepatic impairment patients.[Pubmed:27128228]
Clin Pharmacol Drug Dev. 2014 Jan;3(1):34-42.
Hepatic and renal impairment studies were conducted with ruxolitinib, a JAK1&2 inhibitor that is cleared predominantly by metabolism. Both studies were open label, single-dose studies. Ruxolitinib area under the curve (AUC) was increased by 87%, 28%, and 65%, respectively, in subjects with mild, moderate, and severe hepatic impairment compared to healthy subjects with no correlation between exposure of ruxolitinib and the degree of hepatic impairment. The pharmacodynamics (PD) data were consistent with ruxolitinib pharmacokinetics (PK). The renal impairment study showed a surprising finding. While there was no change in ruxolitinib PK with varying degrees of renal impairment, the PD showed increasing pharmacological activity with increased severity of renal impairment. Analysis of the metabolite exposures revealed that active metabolites contributed to the observed incremental increase in PD activity. The recovery of ruxolitinib in dialysate was negligible. The starting dose of ruxolitinib in subjects with any hepatic impairment or moderate or severe renal impairment should be decreased to 10 mg twice daily (BID) if their platelet counts are between 100 x 10(9) /L and 150 x 10(9) /L. Subjects on dialysis should initiate dosing with a single dose of 15 or 20 mg, based on platelet counts, with dosing only on the days of dialysis.


