Baricitinib phosphateJAK1/JAK2 inhibitor CAS# 1187595-84-1 |
- LY2784544
Catalog No.:BCC2200
CAS No.:1229236-86-5
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Tofacitinib (CP-690550) Citrate
Catalog No.:BCC2189
CAS No.:540737-29-9
- AZ 960
Catalog No.:BCC2197
CAS No.:905586-69-8
- AZD1480
Catalog No.:BCC2191
CAS No.:935666-88-9
- S-Ruxolitinib (INCB018424)
Catalog No.:BCC2201
CAS No.:941685-37-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1187595-84-1 | SDF | Download SDF |
| PubChem ID | 44231848 | Appearance | Powder |
| Formula | C16H20N7O6PS | M.Wt | 469.41 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | INCB028050; LY3009104 | ||
| Solubility | DMSO : ≥ 4.7 mg/mL (10.01 mM) H2O : 1 mg/mL (2.13 mM; ultrasonic and warming and heat to 80°C) *"≥" means soluble, but saturation unknown. | ||
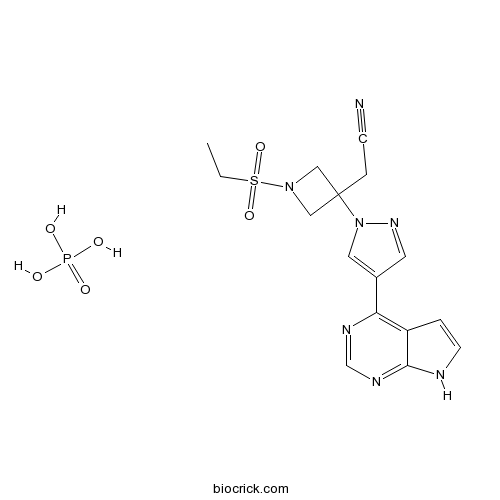
| Chemical Name | 2-[1-ethylsulfonyl-3-[4-(7H-pyrrolo[2,3-d]pyrimidin-4-yl)pyrazol-1-yl]azetidin-3-yl]acetonitrile;phosphoric acid | ||
| SMILES | CCS(=O)(=O)N1CC(C1)(CC#N)N2C=C(C=N2)C3=C4C=CNC4=NC=N3.OP(=O)(O)O | ||
| Standard InChIKey | FBPOWTFFUBBKBB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H17N7O2S.H3O4P/c1-2-26(24,25)22-9-16(10-22,4-5-17)23-8-12(7-21-23)14-13-3-6-18-15(13)20-11-19-14;1-5(2,3)4/h3,6-8,11H,2,4,9-10H2,1H3,(H,18,19,20);(H3,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Baricitinib phosphate is a selective orally bioavailable JAK1/JAK2 inhibitor with IC50 of 5.9 nM and 5.7 nM, respectively.In Vitro:In cell-based assays, Baricitinib (INCB028050) proves to be a potent inhibitor of JAK signaling and function. In PBMCs, Baricitinib inhibits IL-6-stimulated phosphorylation of the canonical substrate STAT3 (pSTAT3) and subsequent production of the chemokine MCP-1 with IC50 values of 44 nM and 40 nM, respectively. In isolated naive T-cells, INCB028050 also inhibits pSTAT3 stimulated by IL-23 (IC50=20 nM). Importantly, this inhibition prevented the production of two pathogenic cytokines (IL-17 and IL-22) produced by Th17 cells-a subtype of helper T cells with demonstrable inflammatory and pathogenic properties-with an IC50 value of 50 nM. In stark contrast, the structurally similar but ineffective JAK1/2 inhibitors INCB027753 and INCB029843 has no significant effect in any of these assays systems when tested at concentrations up to 10 μM[1].In Vivo:Baricitinib (INCB028050) treatment, compares with vehicle, inhibits the increase in hind paw volumes during the 2 wk of treatment by 50% at a dose of 1 mg/kg and >95% at doses of 3 or 10 mg/kg. Because baseline paw volume measurements are taken on treatment day 0-in animals with significant signs of disease-it is possible to have >100% inhibition in animals showing marked improvement in swelling[1]. Baricitinib (0.7 mg/day) treated mice exhibits substantially reduced inflammation as assessed by H&E; staining, reduced CD8 infiltration, and reduced MHC class I and class II expression when compared with vehicle-control treated mice. CD8+NKG2D+ cells, critical effectors of disease in murine and human alopecia areata (AA), are greatly diminished in Baricitinib treated mice compare with vehicle control treated mice[2]. References: | |||||

Baricitinib phosphate Dilution Calculator

Baricitinib phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1303 mL | 10.6517 mL | 21.3033 mL | 42.6067 mL | 53.2583 mL |
| 5 mM | 0.4261 mL | 2.1303 mL | 4.2607 mL | 8.5213 mL | 10.6517 mL |
| 10 mM | 0.213 mL | 1.0652 mL | 2.1303 mL | 4.2607 mL | 5.3258 mL |
| 50 mM | 0.0426 mL | 0.213 mL | 0.4261 mL | 0.8521 mL | 1.0652 mL |
| 100 mM | 0.0213 mL | 0.1065 mL | 0.213 mL | 0.4261 mL | 0.5326 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Direct and indirect inhibition of the JAKs has demonstrated rapid and sustained improvement in clinical measures of disease. Baricitinib phosphate (INCB 028050) is a selective JAK1 and JAK2 inhibitor.
In vitro: Baricitinib phosphate is a selective orally bioavailable JAK1/JAK2 inhibitor with nanomolar potency against JAK1 (5.9 nM) and JAK2 (5.7 nM) and ~10-fold selective versus JAK3 and Tyk2, no inhibition to c-Met and Chk2. INCB028050 inhibits intracellular signaling of multiple proinflammatory cytokines including IL-6 and IL-23 at concentrations, 50 nM [1].
In vivo: Significant efficacy was achieved in the rat adjuvant arthritis model with doses of Baricitinib phosphate providing partial and/or periodic inhibition of JAK1/JAK2 and no inhibition of JAK3. Baricitinib phosphate was also effective in multiple murine models of arthritis, with no evidence of suppression of humoral immunity or adverse hematologic effects [2].
Clinical trial: Several clinical trials have been conducted to investigated the PK, efficacy and DDI of Baricitinib phosphate in healthy people as well as in participants With Rheumatoid Arthritis.
Reference:
[1] Fridman JS, Scherle PA, Collins R, Burn TC, Li Y, Li J, Covington MB, Thomas B, Collier P, Favata MF, Wen X, Shi J, McGee R, Haley PJ, Shepard S, Rodgers JD, Yeleswaram S, Hollis G, Newton RC, Metcalf B, Friedman SM, Vaddi K. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol. 2010;184(9):5298-307.
- Baricitinib (LY3009104, INCB028050)
Catalog No.:BCC2195
CAS No.:1187594-09-7
- PF 184
Catalog No.:BCC6130
CAS No.:1187460-81-6
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- Carabrolactone A
Catalog No.:BCN6063
CAS No.:1187925-30-9
- Carabrolactone B
Catalog No.:BCN6064
CAS No.:1187925-31-0
- Diosbulbin I
Catalog No.:BCN6065
CAS No.:1187951-05-8
- Diosbulbin J
Catalog No.:BCN6066
CAS No.:1187951-06-9
- Ac-Leu-OH
Catalog No.:BCC2967
CAS No.:1188-21-2
- ent-14,16-Epoxy-8-pimarene-3,15-diol
Catalog No.:BCN6067
CAS No.:1188281-98-2
- 7-Hydroxydarutigenol
Catalog No.:BCN6068
CAS No.:1188281-99-3
- 9-Hydroxydarutigenol
Catalog No.:BCN6069
CAS No.:1188282-00-9
- 16-O-Acetyldarutigenol
Catalog No.:BCN6070
CAS No.:1188282-01-0
- 15,16-Di-O-acetyldarutoside
Catalog No.:BCN6071
CAS No.:1188282-02-1
- Ustusol C
Catalog No.:BCN6757
CAS No.:1188398-13-1
- Ustusolate C
Catalog No.:BCN6755
CAS No.:1188398-15-3
Novel Oral Therapies for Psoriasis and Psoriatic Arthritis.[Pubmed:26923915]
Am J Clin Dermatol. 2016 Jun;17(3):191-200.
Several classes of new oral therapy are in use or in development for the treatment of psoriasis. Despite the high efficacy of biologics, new oral therapies remain important as patients generally prefer this mode of administration and they offer an alternative risk-benefit profile. In this review, we discuss the novel modes of action of these drugs, including modulation of cellular pathways involving diverse targets such as Janus kinase, phosphodiesterase 4, sphingosine 1-phosphate, A3 adenosine receptor and rho-associated kinase 2. We review the available evidence around licensed drugs (apremilast) and drugs that are advanced (tofacitinib) or early (ponesimod, baricitinib, peficitinib, INCB039110, CF101, KD025) in the development pipeline. The key limitations of these oral therapies are their modest efficacy profile (apremilast, ponesimod) and the limitations of their safety profile (tofacitinib, ponesimod), while the evidence for the early pipeline drugs are at phase II level only. Potential niches of current unmet needs include apremilast for patients with concomitant psoriatic arthritis, as combination treatments with biologic therapies, and/or for patients in whom multiple biologic therapies have failed due to immunogenicity and secondary inefficacy. The present knowledge gap regarding these novel drugs includes the need for longer clinical trials or observational studies to evaluate safety, and randomised phase III trials for the early pipeline drugs. We conclude that further research and data are necessary to conclusively establish the role of these agents in the current psoriasis treatment paradigm.
Novel therapies for immune-mediated inflammatory diseases: What can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis?[Pubmed:28765121]
Ann Rheum Dis. 2018 Feb;77(2):175-187.
The past three decades have witnessed remarkable advances in our ability to target specific elements of the immune and inflammatory response, fuelled by advances in both biotechnology and disease knowledge. As well as providing superior treatments for immune-mediated inflammatory diseases (IMIDs), such therapies also offer unrivalled opportunities to study the underlying immunopathological basis of these conditions.In this review, we explore recent approaches to the treatment of IMIDs and the insights to pathobiology that they provide. We review novel biologic agents targeting the T-helper 17 axis, including therapies directed towards interleukin (IL)-17 (secukinumab, ixekizumab, bimekizumab), IL-17R (brodalumab), IL-12/23p40 (ustekinumab, briakinumab) and IL-23p19 (guselkumab, tildrakizumab, brazikumab, risankizumab, mirikizumab). We also present an overview of biologics active against type I and II interferons, including sifalumumab, rontalizumab, anifrolumab and fontolizumab. Emerging strategies to interfere with cellular adhesion processes involved in lymphocyte recruitment are discussed, including both integrin blockade (natalizumab, vedolizumab, etrolizumab) and sphingosine-1-phosphate receptor inhibition (fingolimod, ozanimod). We summarise the development and recent application of Janus kinase (JAK) inhibitors in the treatment of IMIDs, including first-generation pan-JAK inhibitors (tofacitinib, baricitinib, ruxolitinib, peficitinib) and second-generation selective JAK inhibitors (decernotinib, filgotinib, upadacitinib). New biologics targeting B-cells (including ocrelizumab, veltuzumab, tabalumab and atacicept) and the development of novel strategies for regulatory T-cell modulation (including low-dose IL-2 therapy and Tregitopes) are also discussed. Finally, we explore recent biotechnological advances such as the development of bispecific antibodies (ABT-122, COVA322), and their application to the treatment of IMIDs.


