4SC-202Class I HDAC inhibitor CAS# 1186222-89-8 |
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Hydrochlorothiazide
Catalog No.:BCC4786
CAS No.:58-93-5
- Miglustat
Catalog No.:BCC5187
CAS No.:72599-27-0
- TPT-260
Catalog No.:BCC5171
CAS No.:769856-81-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1186222-89-8 | SDF | Download SDF |
| PubChem ID | 44217246 | Appearance | Powder |
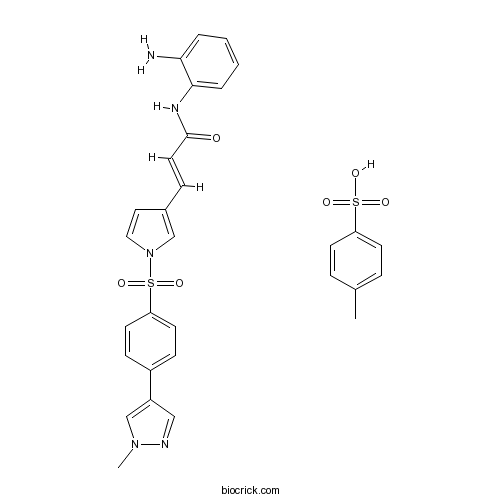
| Formula | C30H29N5O6S2 | M.Wt | 619.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | domatinostat tosylate | ||
| Solubility | DMSO : ≥ 51 mg/mL (82.30 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | (E)-N-(2-aminophenyl)-3-[1-[4-(1-methylpyrazol-4-yl)phenyl]sulfonylpyrrol-3-yl]prop-2-enamide;4-methylbenzenesulfonic acid | ||
| SMILES | CC1=CC=C(C=C1)S(=O)(=O)O.CN1C=C(C=N1)C2=CC=C(C=C2)S(=O)(=O)N3C=CC(=C3)C=CC(=O)NC4=CC=CC=C4N | ||
| Standard InChIKey | IAVXAZDVNICKFJ-ICSBZGNSSA-N | ||
| Standard InChI | InChI=1S/C23H21N5O3S.C7H8O3S/c1-27-16-19(14-25-27)18-7-9-20(10-8-18)32(30,31)28-13-12-17(15-28)6-11-23(29)26-22-5-3-2-4-21(22)24;1-6-2-4-7(5-3-6)11(8,9)10/h2-16H,24H2,1H3,(H,26,29);2-5H,1H3,(H,8,9,10)/b11-6+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 4SC-202 is a selective class I HDAC inhibitor with IC50 of 1.20 μM, 1.12 μM, and 0.57 μM for HDAC1, HDAC2, and HDAC3, respectively. It also displays inhibitory activity against Lysine specific demethylase 1 (LSD1).In Vitro:4SC-202 significantly reduces proliferation of all epithelial and mesenchymal UC cell lines (IC50 0.15-0.51 μM), inhibits clonogenic growth and induces caspase activity[1]. 4SC-202 provokes apoptosis activation in CRC cells, while caspase inhibitors (z-VAD-CHO and z-DVED-CHO) significantly alleviate 4SC-202-exerted cytotoxicity in CRC cells. Meanwhile, 4SC-202 induces dramatic G2-M arrest in CRC cells. Further studies show that AKT activation might be an important resistance factor of 4SC-202. 4SC-202-induced cytotoxicity is dramatically potentiated with serum starvation, AKT inhibition (by perifosine or MK-2206), or AKT1-shRNA knockdown in CRC cells. On the other hand, exogenous expression of constitutively active AKT1 (CA-AKT1) decreases the sensitivity by 4SC-202 in HT-29 cells. Notably, 4SC-202, at a low concentration, enhances oxaliplatin-induced in vitro anti-CRC activity[2]. 4SC-202 treatment induces potent cytotoxic and proliferation-inhibitory activities against established HCC cell lines (HepG2, HepB3, SMMC-7721) and patient-derived primary HCC cells. 4SC-202 induces apoptosis signal-regulating kinase 1 (ASK1) activation, causing it translocation to mitochondria and physical association with Cyp-D[3].In Vivo:Oral gavage of 4SC-202 inhibits HT-29 xenograft growth in nude mice, and when combined with oxaliplatin, its activity is further strengthened[2]. References: | |||||

4SC-202 Dilution Calculator

4SC-202 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.6137 mL | 8.0683 mL | 16.1366 mL | 32.2732 mL | 40.3415 mL |
| 5 mM | 0.3227 mL | 1.6137 mL | 3.2273 mL | 6.4546 mL | 8.0683 mL |
| 10 mM | 0.1614 mL | 0.8068 mL | 1.6137 mL | 3.2273 mL | 4.0341 mL |
| 50 mM | 0.0323 mL | 0.1614 mL | 0.3227 mL | 0.6455 mL | 0.8068 mL |
| 100 mM | 0.0161 mL | 0.0807 mL | 0.1614 mL | 0.3227 mL | 0.4034 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
4SC-202 is a selective, potent and orally available inhibitor of histone deacetylases (HDAC) specific for class I HDAC isoenzymes with an IC50 value of about 1μM [1].
4SC-202 has been reported to selectively inhibit the recombinant class I HDAC isoenzymes with IC50 values of 1.2μM, 1.12μM and 0.57μM for HDAC-1, HDAC-2 and HDAC-3, respectively. In vitro studies, 4SC-202 has been revealed to induce hyperacetylation of histone H3 in a concentration-dependent fashion with an EC50 values of 1.1μM in HeLa and RKO cell lines. In addition, 4SC-202 has been demonstrated to induce a G2/M cell cycle arrest and enhance in sub G1 cells. In other words, 4SC-202 can induce the apoptotic in NSCLS cell lines. 4SC-202 has been noted to suppress proliferative activity in human cancer cell lines with a mean IC50 of 0.7μM.
In vivo studies, 4SC-202 has shown a good tolerability and dose-dependent effect on anti-tumour activity compared with other inhibitors in the A549NSCLC xenograft model and the RKO27 colon carcinoma model [1].
References:
[1] Henning S W, Doblhofer R, Kohlhof H, et al. 178 Preclinical characterization of 4SC-202, a novel isotype specific HDAC inhibitor[J]. European Journal of Cancer Supplements, 2010, 8(7): 61.
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- Tocrifluor T1117
Catalog No.:BCC7401
CAS No.:1186195-59-4
- BU 226 hydrochloride
Catalog No.:BCC6936
CAS No.:1186195-56-1
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
- Trametinib DMSO solvate
Catalog No.:BCC2013
CAS No.:1187431-43-1
- PF 184
Catalog No.:BCC6130
CAS No.:1187460-81-6
- Baricitinib (LY3009104, INCB028050)
Catalog No.:BCC2195
CAS No.:1187594-09-7
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- Carabrolactone A
Catalog No.:BCN6063
CAS No.:1187925-30-9
Pre-clinical characterization of 4SC-202, a novel class I HDAC inhibitor, against colorectal cancer cells.[Pubmed:26831668]
Tumour Biol. 2016 Aug;37(8):10257-67.
Histone deacetylase (HDAC) overactivity in colorectal cancer (CRC) promotes cancer progression. In the current study, we showed that 4SC-202, a novel class I HDAC inhibitor (HDACi), potently inhibited survival and proliferation of primary human colon cancer cells and established CRC lines (HT-29, HCT-116, HT-15, and DLD-1). Yet, the same 4SC-202 treatment was non-cytotoxic to colon epithelial cells where HDAC-1/-2 expressions were extremely low. 4SC-202 provoked apoptosis activation in CRC cells, while caspase inhibitors (z-VAD-CHO and z-DVED-CHO) significantly alleviated 4SC-202-exerted cytotoxicity in CRC cells. Meanwhile, 4SC-202 induced dramatic G2-M arrest in CRC cells. Further studies showed that AKT activation might be an important resistance factor of 4SC-202. 4SC-202-induced cytotoxicity was dramatically potentiated with serum starvation, AKT inhibition (by perifosine or MK-2206), or AKT1-shRNA knockdown in CRC cells. On the other hand, exogenous expression of constitutively active AKT1 (CA-AKT1) decreased the sensitivity by 4SC-202 in HT-29 cells. Notably, 4SC-202, at a low concentration, enhanced oxaliplatin-induced in vitro anti-CRC activity. In vivo, we showed that oral gavage of 4SC-202 inhibited HT-29 xenograft growth in nude mice, and when combined with oxaliplatin, its activity was further strengthened. Together, these pre-clinical results indicate that 4SC-202 may be further investigated as a valuable anti-CRC agent/chemo-adjuvant.
Evaluation of the Therapeutic Potential of the Novel Isotype Specific HDAC Inhibitor 4SC-202 in Urothelial Carcinoma Cell Lines.[Pubmed:27250763]
Target Oncol. 2016 Dec;11(6):783-798.
BACKGROUND: Targeting of class I histone deacetylases (HDACs) exerts antineoplastic actions in various cancer types by modulation of transcription, upregulation of tumor suppressors, induction of cell cycle arrest, replication stress and promotion of apoptosis. Class I HDACs are often deregulated in urothelial cancer. 4SC-202, a novel oral benzamide type HDAC inhibitor (HDACi) specific for class I HDACs HDAC1, HDAC2 and HDAC3 and the histone demethylase LSD1, shows substantial anti-tumor activity in a broad range of cancer cell lines and xenograft tumor models. AIM: The aim of this study was to investigate the therapeutic potential of 4SC-202 in urothelial carcinoma (UC) cell lines. METHODS: We determined dose response curves of 4SC-202 by MTT assay in seven UC cell lines with distinct HDAC1, HDAC2 and HDAC3 expression profiles. Cellular effects were further analyzed in VM-CUB1 and UM-UC-3 cells by colony forming assay, caspase-3/7 assay, flow cytometry, senescence assay, LDH release assay, and immunofluorescence staining. Response markers were followed by quantitative real-time PCR and western blotting. Treatment with the class I HDAC specific inhibitor SAHA (vorinostat) served as a general control. RESULTS: 4SC-202 significantly reduced proliferation of all epithelial and mesenchymal UC cell lines (IC50 0.15-0.51 muM), inhibited clonogenic growth and induced caspase activity. Flow cytometry revealed increased G2/M and subG1 fractions in VM-CUB1 and UM-UC-3 cells. Both effects were stronger than with SAHA treatment. CONCLUSION: Specific pharmacological inhibition of class I HDACs by 4SC-202 impairs UC cell viability, inducing cell cycle disturbances and cell death. Combined inhibition of HDAC1, HDAC2 and HDAC3 seems to be a promising treatment strategy for UC.
4SC-202 activates ASK1-dependent mitochondrial apoptosis pathway to inhibit hepatocellular carcinoma cells.[Pubmed:26773495]
Biochem Biophys Res Commun. 2016 Mar 4;471(2):267-73.
The aim of the present study is to investigate the potential anti-hepatocellular carcinoma (HCC) cell activity by 4SC-202, a novel class I HDAC inhibitor (HDACi). The associated signaling mechanisms were also analyzed. We showed that 4SC-202 treatment induced potent cytotoxic and proliferation-inhibitory activities against established HCC cell lines (HepG2, HepB3, SMMC-7721) and patient-derived primary HCC cells. Further, adding 4SC-202 in HCC cells activated mitochondrial apoptosis pathway, which was evidenced by mitochondrial permeability transition pore (mPTP) opening, cytochrome C cytosol release and caspase-3/-9 activation. Inhibition of this apoptosis pathway, by caspase-3/-9 inhibitors, mPTP blockers, or by shRNA-mediated knockdown of cyclophilin-D (Cyp-D, a key component of mPTP), significantly attenuated 4SC-202-induced HCC cell death and apoptosis. Reversely, over-expression of Cyp-D enhanced 4SC-202's sensitivity in HCC cells. Further studies showed that 4SC-202 induced apoptosis signal-regulating kinase 1 (ASK1) activation, causing it translocation to mitochondria and physical association with Cyp-D. This mitochondrial ASK1-Cyp-D complexation appeared required for mediating 4SC-202-induced apoptosis activation. ASK1 stable knockdown by targeted-shRNAs largely inhibited 4SC-202-induced mPTP opening, cytochrome C release, and following HCC cell apoptotic death. Together, we suggest that 4SC-202 activates ASK1-dependent mitochondrial apoptosis pathway to potently inhibit human HCC cells.


