BU 226 hydrochlorideLigand at I2 imidazoline sites,high affinity and selectivity CAS# 1186195-56-1 |
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Verteporfin
Catalog No.:BCC3690
CAS No.:129497-78-5
- Methylcobalamin
Catalog No.:BCC5188
CAS No.:13422-55-4
- Miglustat hydrochloride
Catalog No.:BCC5186
CAS No.:210110-90-0
- Sulfasalazine
Catalog No.:BCC2545
CAS No.:599-79-1
- SC 144
Catalog No.:BCC1171
CAS No.:895158-95-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

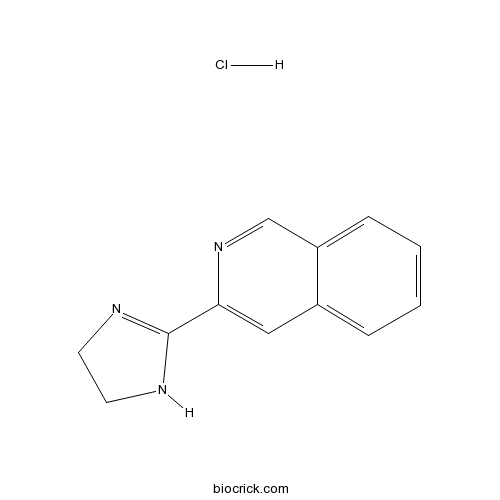
| Cas No. | 1186195-56-1 | SDF | Download SDF |
| PubChem ID | 56972194 | Appearance | Powder |
| Formula | C12H12ClN3 | M.Wt | 233.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | 3-(4,5-dihydro-1H-imidazol-2-yl)isoquinoline;hydrochloride | ||
| SMILES | C1CN=C(N1)C2=CC3=CC=CC=C3C=N2.Cl | ||
| Standard InChIKey | NIGHNKWLMSRCOZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C12H11N3.ClH/c1-2-4-10-8-15-11(7-9(10)3-1)12-13-5-6-14-12;/h1-4,7-8H,5-6H2,(H,13,14);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | A high affinity ligand for I2 imidazoline sites (Ki = 2.7 nM) with very high selectivity (> 2000-fold) over α2-adrenoceptors (Ki = 6700 nM). |

BU 226 hydrochloride Dilution Calculator

BU 226 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.279 mL | 21.395 mL | 42.7899 mL | 85.5798 mL | 106.9748 mL |
| 5 mM | 0.8558 mL | 4.279 mL | 8.558 mL | 17.116 mL | 21.395 mL |
| 10 mM | 0.4279 mL | 2.1395 mL | 4.279 mL | 8.558 mL | 10.6975 mL |
| 50 mM | 0.0856 mL | 0.4279 mL | 0.8558 mL | 1.7116 mL | 2.1395 mL |
| 100 mM | 0.0428 mL | 0.2139 mL | 0.4279 mL | 0.8558 mL | 1.0697 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
BU 226 hydrochloride is a selective ligand of imidazoline2 receptor [1].
Imidazoline receptor is the primary receptor for clonidine and other imidazolines. Imidazoline2 receptor (I2 receptor) is an allosteric binding site of monoamine oxidase and plays an important role in neuroprotection and pain modulation.
BU 226 hydrochloride is a selective ligand of I2 receptor. BU226 exhibited high affinity for I2 receptor with Ki value of 1.4 nM and displayed 380-fold selectivity against I1 receptor. Also, BU226 showed low affinity for α2-adrenoceptor. In rat brain or kidney membranes, BU226 showed affinity for I1, I2 receptors and α2-adrenoceptor with IC50 value of 534.5 nM and Ki values of 2.7 and 6700 nM, respectively [1]. In pig brain, BU226 displaced of 2BFI with Ki value of 44.7 nM, which suggested the presence of imidazoline I2 binding site [2].
In male Hooded Lister rats, BU226(1.6-7.0 mg/kg) potently substituted for 2-BFI with ED50 value of 3.2 mg/kg in a dose-dependent way [3].
References:
[1]. Hudson AL, Gough R, Tyacke R, et al. Novel selective compounds for the investigation of imidazoline receptors. Ann N Y Acad Sci, 1999, 881: 81-91.
[2]. Anderson NJ, Lupo PA, Nutt DJ, et al. Characterisation of imidazoline I2 binding sites in pig brain. Eur J Pharmacol, 2005, 519(1-2): 68-74.
[3]. MacInnes N, Handley SL. Characterization of the discriminable stimulus produced by 2-BFI: effects of imidazoline I(2)-site ligands, MAOIs, beta-carbolines, agmatine and ibogaine. Br J Pharmacol, 2002, 135(5): 1227-1234.
- Vitexdoin A
Catalog No.:BCN4089
CAS No.:1186021-77-1
- VD3-D6
Catalog No.:BCC4076
CAS No.:118584-54-6
- NVP-BVU972
Catalog No.:BCC3828
CAS No.:1185763-69-2
- Zileuton sodium
Catalog No.:BCC4216
CAS No.:118569-21-4
- Floribundone 1
Catalog No.:BCN4726
CAS No.:118555-84-3
- Phaseoloidin
Catalog No.:BCN8451
CAS No.:118555-82-1
- Boc-Orn(2-Cl-Z)-OH
Catalog No.:BCC3428
CAS No.:118554-00-0
- H-Orn(2-Cl-Z)-OH
Catalog No.:BCC3002
CAS No.:118553-99-4
- Baohuoside V
Catalog No.:BCN2887
CAS No.:118544-18-6
- Icaritin
Catalog No.:BCN5352
CAS No.:118525-40-9
- Sagittatoside C
Catalog No.:BCN3059
CAS No.:118525-37-4
- Sagittatoside B
Catalog No.:BCN2357
CAS No.:118525-36-3
- Tocrifluor T1117
Catalog No.:BCC7401
CAS No.:1186195-59-4
- MTEP hydrochloride
Catalog No.:BCC1780
CAS No.:1186195-60-7
- NPE-caged-proton
Catalog No.:BCC7698
CAS No.:1186195-63-0
- ALW-II-41-27
Catalog No.:BCC1350
CAS No.:1186206-79-0
- 4SC-202
Catalog No.:BCC5359
CAS No.:1186222-89-8
- Epivogeloside
Catalog No.:BCN6060
CAS No.:118627-52-4
- TMN 355
Catalog No.:BCC6121
CAS No.:1186372-20-2
- Evacetrapib (LY2484595)
Catalog No.:BCC2329
CAS No.:1186486-62-3
- 11-Hydroxycodaphniphylline
Catalog No.:BCN6061
CAS No.:1186496-68-3
- H-Cys(Me)-OH
Catalog No.:BCC2908
CAS No.:1187-84-4
- Resminostat hydrochloride
Catalog No.:BCC1888
CAS No.:1187075-34-8
- Cuniloside B
Catalog No.:BCN6062
CAS No.:1187303-40-7
Novel selective compounds for the investigation of imidazoline receptors.[Pubmed:10415900]
Ann N Y Acad Sci. 1999 Jun 21;881:81-91.
Over several years our group has sought to synthesize and identify selective ligands for imidazoline (I) receptors, in particular the I2 binding site. As a consequence, [3H]2-(2-benzofuranyl)-2-imidazoline (2BFI) has proved extremely useful for binding and autoradiographic studies. More recently we have synthesized a BU series of compounds and examined these for their affinities for both I1 and I2 binding sites. BU224 (2-(4,5-dihydroimidaz-2-yl)-quinoline) shows high affinity for I2 receptors with a Ki of 2.1 nM. BU226 (2-(4,5-dihydroimidaz-2-yl)-isoquinoline) demonstrated slightly higher affinity (Ki 1.4 nM) for I2 receptors, but overall BU224 displayed greater selectivity for I2 over I1 receptors (832-fold) than BU226 (380-fold). Both compounds showed low (microM) affinity for alpha 2-adrenoceptors. Given BU224's ability to cross the blood brain barrier, we predict that its in vivo effects are likely to be mediated via I2 receptors. Brain dialysis revealed BU224 to dose dependently (0-20 mg/kg i.p.) elevate basal noradrenaline in rat frontal cortex and basal dopamine in striatum. In a rat model of opiate withdrawal, behavioral studies showed that BU224 (10 mg/kg, s.c.) was able to reduce acute weight loss and diarrhea, but not the number of wet dog shakes associated with the withdrawal syndrome.


