Risedronate SodiumFPP synthase inhibitor CAS# 115436-72-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 115436-72-1 | SDF | Download SDF |
| PubChem ID | 68739 | Appearance | Powder |
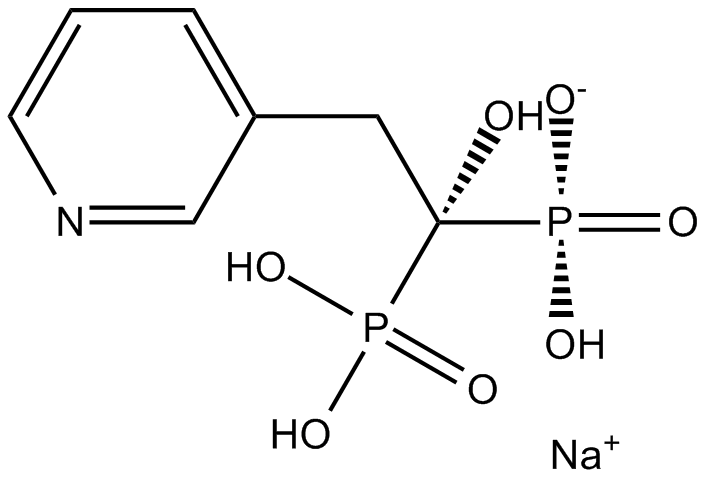
| Formula | C7H10NNaO7P2 | M.Wt | 305.09 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Risedronic Acid Sodium | ||
| Solubility | H2O : 8.33 mg/mL (27.21 mM; Need ultrasonic) DMSO : < 1 mg/mL (insoluble or slightly soluble) | ||
| Chemical Name | sodium;hydroxy-(1-hydroxy-1-phosphono-2-pyridin-3-ylethyl)phosphinate | ||
| SMILES | C1=CC(=CN=C1)CC(O)(P(=O)(O)O)P(=O)(O)[O-].[Na+] | ||
| Standard InChIKey | DRFDPXKCEWYIAW-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C7H11NO7P2.Na/c9-7(16(10,11)12,17(13,14)15)4-6-2-1-3-8-5-6;/h1-3,5,9H,4H2,(H2,10,11,12)(H2,13,14,15);/q;+1/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Orally active biphosphonate that inhibits farnesyl diphosphate (FPP) synthase (IC50 = 100 nM). Exhibits antiproliferative and proapoptotic activity in numerous tumor cell lines and inhibits osteoclast-mediated bone resorption in vivo. |

Risedronate Sodium Dilution Calculator

Risedronate Sodium Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2777 mL | 16.3886 mL | 32.7772 mL | 65.5544 mL | 81.943 mL |
| 5 mM | 0.6555 mL | 3.2777 mL | 6.5554 mL | 13.1109 mL | 16.3886 mL |
| 10 mM | 0.3278 mL | 1.6389 mL | 3.2777 mL | 6.5554 mL | 8.1943 mL |
| 50 mM | 0.0656 mL | 0.3278 mL | 0.6555 mL | 1.3111 mL | 1.6389 mL |
| 100 mM | 0.0328 mL | 0.1639 mL | 0.3278 mL | 0.6555 mL | 0.8194 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Orally active biphosphonate that inhibits farnesyl diphosphate (FPP) synthase. Exhibits antiproliferative and proapoptotic activity in numerous tumor cell lines and inhibits osteoclast-mediated bone reabsorption in vivo.
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- EIPA
Catalog No.:BCC7672
CAS No.:1154-25-2
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
- Marbofloxacin hydrochloride
Catalog No.:BCC4250
CAS No.:115551-26-3
- 2'-Hydroxygenistein
Catalog No.:BCN6036
CAS No.:1156-78-1
- 4-Androstenediol
Catalog No.:BCC8692
CAS No.:1156-92-9
Enteric-coated tablet of risedronate sodium in combination with phytic acid, a natural chelating agent, for improved oral bioavailability.[Pubmed:26594027]
Eur J Pharm Sci. 2016 Jan 20;82:45-51.
The oral bioavailability (BA) of Risedronate Sodium (RS), an antiresorptive agent, is less than 1% due to its low membrane permeability as well as the formation of non-absorbable complexes with multivalent cations such as calcium ion (Ca(2+)) in the gastrointestinal tract. In the present study, to increase oral BA of the bisphosphonate, a novel enteric-coated tablet (ECT) dosage form of RS in combination with phytic acid (IP6), a natural chelating agent recognized as safe, was formulated. The chelating behavior of IP6 against Ca(2+), including a stability constant for complex formulation was characterized using the continuous variation method. Subsequently, in vitro dissolution profile and in vivo pharmacokinetic profile of the novel ECT were evaluated comparatively with that of the marketed product (Altevia, Sanofi, US), an ECT containing ethylenediaminetetraacetic acid (EDTA) as a chelating agent, in beagle dogs. The logarithm of stability constant for Ca(2+)-IP6 complex, an equilibrium constant approximating the strength of the interaction between two chemicals to form complex, was 19.05, which was 3.9-fold (p<0.05) and 1.7-fold (p<0.05) higher than those of Ca(2+)-RS and Ca(2+)-EDTA complexes. The release profile of RS from both enteric-coated dosage forms was equivalent, regardless of the type of chelating agent. An in vivo absorption study in beagle dogs revealed that the maximum plasma concentration and area under the curve of RS after oral administration of IP6-containing ECT were approximately 7.9- (p<0.05) and 5.0-fold (p<0.05) higher than those of the marketed product at the same dose (35mg as RS). Therefore, our study demonstrates the potential usefulness of the ECT system in combination with IP6 for an oral therapy with the bisphosphonate for improved BA.
Biodegradable intranasal nanoparticulate drug delivery system of risedronate sodium for osteoporosis.[Pubmed:25625496]
Drug Deliv. 2016 Sep;23(7):2428-2438.
CONTEXT: Osteoporosis (OP) is the most common metabolic bone disease predominantly found in elderly people. It is associated with reduced bone mineral density, results in a higher probability of fractures, especially of the hip, vertebrae, and distal radius. Worldwide prevalence of OP is considered a serious public health concern. OBJECTIVE: The purpose of the present work was to develop and evaluate polymeric nanoparticles (NPs) of Risedronate Sodium (RIS) for the treatment of OP using intranasal (IN) route in order to reduce peripheral toxic effects. MATERIALS AND METHODS: Polymeric NPs of RIS were prepared by nanoprecipitation methods. Formulations were developed and evaluated in context to in vitro drug release, ex vivo permeation, in vivo study, and biochemical studies. RESULTS AND DISCUSSIONS: The particles size, entrapment efficiency (EE) (%), and loading capacity (LC) (%) of optimized formulations were found to be 127.84 +/- 6.33 nm, 52.65 +/- 5.21, and 10.57 +/- 1.48, respectively. Release kinetics showed diffusion-controlled, Fickian release pattern. Ex vivo permeation study showed RIS from PLGA-NPs permeated significantly (p < 0.05) through nasal mucosa. In vivo study showed a marked difference in micro-structure (trabeculae) in bone internal environment. Biochemical estimation of treated group and RIS PLGA indicated a significant recovery (p < 0.01) as compared with the toxic group. CONCLUSION: Polymeric NPs of RIS were prepared successfully using biodegradable polymer (PLGA). Intranasal delivery showed a good result in in vivo study. Thus PLGA-NPs have great potential for delivering the RIS for the treatment and prevention of OP after clinical evaluation in near future.
Comparative permeability studies with radioactive and nonradioactive risedronate sodium from self-microemulsifying drug delivery system and solution.[Pubmed:25285358]
Drug Dev Ind Pharm. 2015;41(9):1493-8.
The purpose of this work is to prepare a self-microemulsifying drug delivery system (SMEDDS) for Risedronate Sodium (RSD) and to compare the permeability with RSD solution. The solubility of RSD was determined in different vehicles. Phase diagrams were constructed to determine the optimum concentration of oil, surfactant, and cosurfactant. RSD SMEDDS was prepared by using a mixture of soybean oil, cremophor EL, span 80, and transcutol (2.02:7.72:23.27:61.74, w/w, respectively). The prepared RSD SMEDDS was characterized by droplet size value. In vitro Caco-2 cell permeability studies were performed for SMEDDS and solution of radioactive ((99 m)Tc-labeled RSD) and nonradioactive RSD. The experimental results indicated that RSD SMEDDS has good stability and its droplet size is between 216.68 +/- 3.79 and 225.26 +/- 7.65 during stability time. In addition, RSD SMEDDS has higher permeability value than the RSD solution for both radioactive and nonradioactive experiments. The results illustrated the potential use of SMEDDS for delivery of poorly absorbed RSD.
Lowering bone mineral affinity of bisphosphonates as a therapeutic strategy to optimize skeletal tumor growth inhibition in vivo.[Pubmed:18974139]
Cancer Res. 2008 Nov 1;68(21):8945-53.
Bisphosphonates bind avidly to bone mineral and are potent inhibitors of osteoclast-mediated bone destruction. They also exhibit antitumor activity in vitro. Here, we used a mouse model of human breast cancer bone metastasis to examine the effects of risedronate and NE-10790, a phosphonocarboxylate analogue of the bisphosphonate risedronate, on osteolysis and tumor growth. Osteolysis was measured by radiography and histomorphometry. Tumor burden was measured by fluorescence imaging and histomorphometry. NE-10790 had a 70-fold lower bone mineral affinity compared with risedronate. It was 7-fold and 8,800-fold less potent than risedronate at reducing, respectively, breast cancer cell viability in vitro and bone loss in ovariectomized animals. We next showed that risedronate given at a low dosage in animals bearing human B02-GFP breast tumors reduced osteolysis by inhibiting bone resorption, whereas therapy with higher doses also inhibited skeletal tumor burden. Conversely, therapy with NE-10790 substantially reduced skeletal tumor growth at a dosage that did not inhibit osteolysis, a higher dosage being able to also reduce bone destruction. The in vivo antitumor activity of NE-10790 was restricted to bone because it did not inhibit the growth of subcutaneous B02-GFP tumor xenografts nor the formation of B16-F10 melanoma lung metastases. Moreover, NE-10790, in combination with risedronate, reduced both osteolysis and skeletal tumor burden, whereas NE-10790 or risedronate alone only decreased either tumor burden or osteolysis, respectively. In conclusion, our study shows that decreasing the bone mineral affinity of bisphosphonates is an effective therapeutic strategy to inhibit skeletal tumor growth in vivo.
Recent advances in understanding the mechanism of action of bisphosphonates.[Pubmed:16650801]
Curr Opin Pharmacol. 2006 Jun;6(3):307-12.
Bisphosphonates (BPs) are widely used in the treatment of diseases associated with excessive osteoclast-mediated bone resorption, such as osteoporosis. Although several years ago the molecular target of the potent nitrogen-containing BPs (N-BPs) was identified as farnesyl diphosphate synthase, an enzyme in the mevalonate pathway, recent data have shed new light on the precise mechanism of inhibition and demonstrated that the acute-phase reaction, an adverse effect of N-BPs, is also caused by inhibition of this enzyme. In addition, the identification of BP analogues that inhibit different enzymes in the mevalonate pathway could lead to the development of novel inhibitors of bone resorption with potential applications in the treatment of bone disease.


