EIPAInhibits TRPP3-mediated currents; also inhibits the Na+/H+ exchanger (NHE) CAS# 1154-25-2 |
- Tenofovir
Catalog No.:BCC2500
CAS No.:147127-20-6
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Tenofovir hydrate
Catalog No.:BCC4261
CAS No.:206184-49-8
- Dapivirine (TMC120)
Catalog No.:BCC3882
CAS No.:244767-67-7
- Zidovudine
Catalog No.:BCC5024
CAS No.:30516-87-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1154-25-2 | SDF | Download SDF |
| PubChem ID | 1795 | Appearance | Powder |
| Formula | C11H8ClN7O | M.Wt | 289.7 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Ethylisopropyl amiloride | ||
| Solubility | DMSO : 140 mg/mL (467.04 mM; Need ultrasonic) | ||
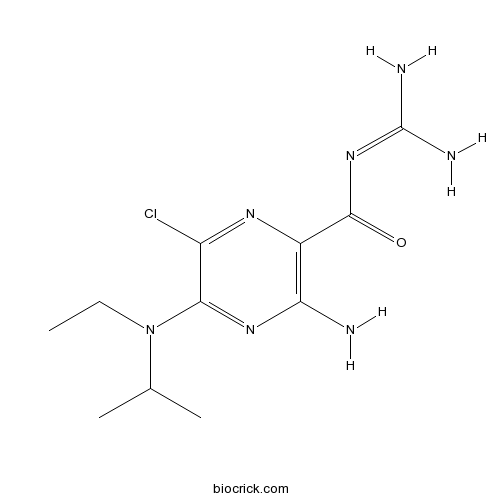
| Chemical Name | 3-amino-6-chloro-N-(diaminomethylidene)-5-[ethyl(propan-2-yl)amino]pyrazine-2-carboxamide | ||
| SMILES | CCN(C1=NC(=C(N=C1Cl)C(=O)N=C(N)N)N)C(C)C | ||
| Standard InChIKey | QDERNBXNXJCIQK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C11H18ClN7O/c1-4-19(5(2)3)9-7(12)16-6(8(13)17-9)10(20)18-11(14)15/h5H,4H2,1-3H3,(H2,13,17)(H4,14,15,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | TRPP3 channel inhibitor (IC50 = 10.5 μM). Inhibits the Na+/H+ exchanger (NHE). Derivative of amiloride. |

EIPA Dilution Calculator

EIPA Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.4518 mL | 17.2592 mL | 34.5185 mL | 69.0369 mL | 86.2962 mL |
| 5 mM | 0.6904 mL | 3.4518 mL | 6.9037 mL | 13.8074 mL | 17.2592 mL |
| 10 mM | 0.3452 mL | 1.7259 mL | 3.4518 mL | 6.9037 mL | 8.6296 mL |
| 50 mM | 0.069 mL | 0.3452 mL | 0.6904 mL | 1.3807 mL | 1.7259 mL |
| 100 mM | 0.0345 mL | 0.1726 mL | 0.3452 mL | 0.6904 mL | 0.863 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
EIPA is a TRPP3 channel inhibitor with an IC50 of 10.5 μM. EIPA also inhibits Na+/H+-exchanger (NHE) and macropinocytosis.
In Vitro:In the presence of 500 μM amiloride, 100 μM EIPA, 10 μM benzamil, and 10 μM phenamil, 45Ca2+ uptake decreases from 79±9 to 46±4 (58% remaining), 27±4 (34%), 29±5 (37%), and 38±4 (48%) pmol/oocyte/30 min (n=6, P=0.008), respectively. It is found that EIPA, benzamil, and phenamil rapidly and reversibly block Ca2+-activated TRPP3 channel activation at -50 mV, with IC50s of 143±8 (n=36), 10.5±2.2 (n=28), 1.1±0.3 (n=30), and 0.14±0.04 μM (n=25), respectively[1]. The number of autophagic vacuoles increases dramatically in the HAE and HPE groups after EIPA treatment compare with the HAN and HPN groups. EIPA regulates the initiation and maturation of the autophagy associated with amino acids in IEC-18 cells[2]. In addition, the uptake of cinnamoylphenazine (CA-PZ) and neutral red (NR) is inhibited by EIPA[3].
References:
[1]. Dai XQ, et al. Inhibition of TRPP3 channel by amiloride and analogs. Mol Pharmacol. 2007 Dec;72(6):1576-85.
[2]. Shi H, et al. Na+/H+ Exchanger Regulates Amino Acid-Mediated Autophagy in Intestinal Epithelial Cells. Cell Physiol Biochem. 2017;42(6):2418-2429.
[3]. Zhu BY, et al. A new HDAC inhibitor cinnamoylphenazine shows antitumor activity in association with intensive macropinocytosis.
- Kanshone B
Catalog No.:BCN7700
CAS No.:115370-61-1
- 2,2-Dimethyl-6-phenylpyrano[3,4-b]pyran-8-one
Catalog No.:BCN7280
CAS No.:1153624-36-2
- Kanshone A
Catalog No.:BCN7279
CAS No.:115356-18-8
- Siguazodan
Catalog No.:BCC6954
CAS No.:115344-47-3
- NAN-190 hydrobromide
Catalog No.:BCC6693
CAS No.:115338-32-4
- Dihydroniloticin
Catalog No.:BCN6032
CAS No.:115334-05-9
- Phellochin
Catalog No.:BCN6031
CAS No.:115334-04-8
- Isosalvipuberulin
Catalog No.:BCN6030
CAS No.:115321-32-9
- 3-Bromo-N-phenylcarbazole
Catalog No.:BCN2260
CAS No.:1153-85-1
- A 1120
Catalog No.:BCC7775
CAS No.:1152782-19-8
- Dofetilide
Catalog No.:BCC3770
CAS No.:115256-11-6
- VX-661
Catalog No.:BCC1241
CAS No.:1152311-62-0
- Molidustat (BAY85-3934)
Catalog No.:BCC6412
CAS No.:1154028-82-6
- Niloticin
Catalog No.:BCN6033
CAS No.:115404-57-4
- Risedronate Sodium
Catalog No.:BCC2501
CAS No.:115436-72-1
- Sootepin D
Catalog No.:BCN6034
CAS No.:1154518-97-4
- Glaucin B
Catalog No.:BCN6035
CAS No.:115458-73-6
- 5,6,7,7a-Tetrahydrothieno[3,2-c]pyridine-2(4H)-one hydrochloride
Catalog No.:BCC8721
CAS No.:115473-15-9
- Z-Glu-OH
Catalog No.:BCC2781
CAS No.:1155-62-0
- H-Lys(Z)-OH
Catalog No.:BCC2986
CAS No.:1155-64-2
- (2-Amino-1-hydroxyethyl)phosphonic acid
Catalog No.:BCN1613
CAS No.:115511-00-7
- Fmoc-Cys(Trt)-Opfp
Catalog No.:BCC3480
CAS No.:115520-21-3
- BI 224436
Catalog No.:BCC5531
CAS No.:1155419-89-8
- Marbofloxacin
Catalog No.:BCC2510
CAS No.:115550-35-1
SLC4A11 is an EIPA-sensitive Na(+) permeable pHi regulator.[Pubmed:23864606]
Am J Physiol Cell Physiol. 2013 Oct 1;305(7):C716-27.
Slc4a11, a member of the solute linked cotransporter 4 family that is comprised predominantly of bicarbonate transporters, was described as an electrogenic 2Na(+)-B(OH)4(-) (borate) cotransporter and a Na(+)-2OH(-) cotransporter. The goal of the current study was to confirm and/or clarify the function of SLC4A11. In HEK293 cells transfected with SLC4A11 we tested if SLC4A11 is a: 1) Na(+)-HCO3(-) cotransporter, 2) Na(+)-OH(-)(H(+)) transporter, and/or 3) Na(+)-B(OH)4(-) cotransporter. CO2/HCO3(-) perfusion yielded no significant differences in rate or extent of pHi changes or Na(+) flux in SLC4A11-transfected compared with control cells. Similarly, in CO2/HCO3(-), acidification on removal of Na(+) and alkalinization on Na(+) add back were not significantly different between control and transfected indicating that SLC4A11 does not have Na(+)-HCO3(-) cotransport activity. In the absence of CO2/HCO3(-), SLC4A11-transfected cells showed higher resting intracelllular Na(+) concentration ([Na(+)]i; 25 vs. 17 mM), increased NH4(+)-induced acidification and increased acid recovery rate (160%) after an NH4 pulse. Na(+) efflux and influx were faster (80%) following Na(+) removal and add back, respectively, indicative of Na(+)-OH(-)(H(+)) transport by SLC4A11. The increased alkalinization recovery was confirmed in NHE-deficient PS120 cells demonstrating that SLC4A11 is a bonafide Na(+)-OH(-)(H(+)) transporter and not an activator of NHEs. SLC4A11-mediated H(+) efflux is inhibited by 5-(N-ethyl-N-isopropyl) amiloride (EIPA; EC50: 0.1 muM). The presence of 10 mM borate did not alter dpHi/dt or DeltapH during a Na(+)-free pulse in SLC4A11-transfected cells. In summary our results show that SLC4A11 is not a bicarbonate or borate-linked transporter but has significant EIPA-sensitive Na(+)-OH(-)(H(+)) and NH4(+) permeability.
Experimental Study of the Effects of EIPA, Losartan, and BQ-123 on Electrophysiological Changes Induced by Myocardial Stretch.[Pubmed:25985899]
Rev Esp Cardiol (Engl Ed). 2015 Dec;68(12):1101-10.
INTRODUCTION AND OBJECTIVES: Mechanical response to myocardial stretch has been explained by various mechanisms, which include Na(+)/H(+) exchanger activation by autocrine-paracrine system activity. Drug-induced changes were analyzed to investigate the role of these mechanisms in the electrophysiological responses to acute myocardial stretch. METHODS: Multiple epicardial electrodes and mapping techniques were used to analyze changes in ventricular fibrillation induced by acute myocardial stretch in isolated perfused rabbit hearts. Four series were studied: control (n = 9); during perfusion with the angiotensin receptor blocker losartan (1 muM, n = 8); during perfusion with the endothelin A receptor blocker BQ-123 (0.1 muM, n = 9), and during perfusion with the Na(+)/H(+) exchanger inhibitor EIPA (5-[N-ethyl-N-isopropyl]-amiloride) (1 muM, n = 9). RESULTS: EIPA attenuated the increase in the dominant frequency of stretch-induced fibrillation (control=40.4%; losartan=36% [not significant]; BQ-123=46% [not significant]; and EIPA=22% [P<.001]). During stretch, the activation maps were less complex (P<.0001) and the spectral concentration of the arrhythmia was greater (greater regularity) in the EIPA series: control=18 (3%); EIPA = 26 (9%) (P < .02); losartan=18 (5%) (not significant); and BQ-123=18 (4%) (not significant). CONCLUSIONS: The Na(+)/H(+) exchanger inhibitor EIPA attenuated the electrophysiological effects responsible for the acceleration and increased complexity of ventricular fibrillation induced by acute myocardial stretch. The angiotensin II receptor antagonist losartan and the endothelin A receptor blocker BQ-123 did not modify these effects.
Acidosis and 5-(N-ethyl-N-isopropyl)amiloride (EIPA) Attenuate Zinc/Kainate Toxicity in Cultured Cerebellar Granule Neurons.[Pubmed:26547075]
Biochemistry (Mosc). 2015 Aug;80(8):1065-72.
Cultured cerebellar granule neurons (CGNs) are resistant to the toxic effect of ZnCl2 (0.005 mM, 3 h) and slightly sensitive to the effect of kainate (0.1 mM, 3 h). Simultaneous treatment of CGNs with kainate and ZnCl2 caused intensive neuronal death, which was attenuated by external acidosis (pH 6.5) or 5-(N-ethyl-N-isopropyl)amiloride (EIPA, Na+/H+ exchange blocker, 0.03 mM). Intracellular zinc and calcium ion concentrations ([Zn2+]i and [Ca2+]i) were increased under the toxic action of kainate + ZnCl2, this effect being significantly decreased on external acidosis and increased in case of EIPA addition. Neuronal Zn2+ imaging demonstrated that EIPA increases the cytosolic concentration of free Zn2+ on incubation in Zn2+-containing solution. These data imply that acidosis reduces ZnCl2/kainate toxic effects by decreasing Zn2+ entry into neurons, and EIPA prevents zinc stores from being overloaded with zinc.
An inhibitor of Na(+)/H(+) exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl(-) concentration via DIDS-sensitive pathways.[Pubmed:23075671]
Cell Physiol Biochem. 2012;30(5):1241-53.
BACKGROUND/AIMS: Tumor cells produce a large amount of acidic metabolites due to their high metabolic condition. However, cytosolic pH (pH(c)) of tumor cells is identical to or even slightly higher than that of normal cells. To maintain pH(c) at a normal or higher level, tumor cells would have to have higher expression and/or activity of H(+) transporting systems than normal cells. The purpose of the present study was to identify effects of ethyl-isopropyl amiloride (EIPA, an inhibitor of Na(+)/H(+) exchanger (NHE)) on proliferation of human gastric cancer MKN28 cells. METHODS: Effects of EIPA on proliferation, pH(c), [Cl(-)](c) and expression of proteins regulating cell cycle and MAPKs were studied in MKN28 expressing NHE exposed to EIPA for 48 h. RESULTS: EIPA suppressed proliferation of MKN28 cells by causing G(0)/G(1) arrest without any significant effects on pH(c), but associated with reduction of [Cl(-)](c). Although EIPA alone had no effects on pH(c), EIPA co-applied with DIDS (an inhibitor of Cl(-)/HCO(3)(-) exchangers; i.e., anion exchanger (AE) and Na+-driven Cl(-)/HCO(3)(-) exchanger (NDCBE)) reduced pH(c), suggesting that DIDS-sensitive Cl(-)/HCO(3)(-) transporters such as AE and/or NDCBE keep pH(c) normal by stimulating HCO(3)(-) uptake coupled with Cl(-) release under an NHE-inhibited condition. EIPA-induced lowered [Cl(-)](c) up-regulated expression of p21associated with phosphorylation of MAPKs, suppressing proliferation associated with G(0)/G(1) arrest. CONCLUSIONS : EIPA suppressed proliferation of MKN28 cells through up-regulation of p21 expression via reduction of [Cl(-)](c) as a result from DIDS-sensitive Cl(-)/HCO(3)(-) exchanger-mediated compensation for keeping pH(c) normal under an NHE-inhibited condition. This is the first study revealing that an NHE inhibitor suppressed the proliferation of cancer cells by reducing [Cl(-)](c) but not pH(c).
Functional role of Na+/H+ exchanger in Ca2+ influx mediated via human endothelin type A receptor stably expressed in Chinese hamster ovary cells.[Pubmed:18678984]
J Pharmacol Sci. 2008 Aug;107(4):456-9. Epub 2008 Aug 2.
This study examines the functional role of Na+/H+ exchanger (NHE) in Ca2+ influx mediated by human endothelin type A receptor (ET(A)R) expressed in Chinese hamster ovary (CHO) cells. Endothelin-1 (ET-1) increased extracellular acidification rate (ECAR), which was abolished by 5-(N-ethyl-N-isopropyl)amiloride (EIPA), an NHE inhibitor. EIPA and KB-R7943, a Na+/Ca2+ exchanger (NCX) inhibitor, inhibited ET-1-induced sustained increases in intracellular Ca2+ concentration ([Ca2+]i), and EIPA had no effect on [Ca2+]i after KB-R7943 treatment. ET-1-elicited sustained [Ca2+]i increase was suppressed by reducing extracellular Na+ concentration. These results suggest that possible coupling of NHE with NCX via Na+ transport is involved in ET(A)R-mediated sustained [Ca2+]i increase.
Inhibition of TRPP3 channel by amiloride and analogs.[Pubmed:17804601]
Mol Pharmacol. 2007 Dec;72(6):1576-85.
TRPP3, a member of the transient receptor potential (TRP) superfamily of cation channels, is a Ca2+-activated channel permeable to Ca2+, Na+, and K+. TRPP3 has been implicated in sour tasting in bipolar cells of tongue and in regulation of pH-sensitive action potential in spinal cord neurons. TRPP3 is also present in excitable and nonexcitable cells of other tissues, including retina, brain, heart, testis, and kidney, with unknown functions. In this study, we examined the functional modulation of TRPP3 channel by amiloride and its analogs, known to inhibit several ion channels and transporters and respond to all taste stimuli, using Xenopus laevis oocyte expression, electrophysiology, and radiotracer measurements. We found that amiloride and its analogs inhibit TRPP3 channel activities with different affinities. Radiolabeled (45)Ca2+ uptake showed that TRPP3-mediated Ca2+ transport was inhibited by amiloride, phenamil, benzamil, and 5-(N-ethyl-N-isopropyl)amiloride (EIPA). Two-microelectrode voltage clamp experiments revealed that TRPP3-mediated Ca2+-activated currents are substantially inhibited by amiloride analogs, in an order of potency of phenamil > benzamil > EIPA > amiloride, with IC50 values of 0.14, 1.1, 10.5, and 143 microM, respectively. The inhibition potency positively correlated with the size of inhibitors. Using cell-attached patch clamping, we showed that the amiloride analogs decrease the open probability and mean open time but have no effect on single-channel conductance. Study of inhibition by phenamil in the presence of previously reported inhibitor tetrapentylammonium indicates that amiloride and organic cation inhibitors compete for binding the same site on TRPP3. TRPP3 may contribute to previously reported in vivo amiloride-sensitive cation transport.


