NelfinavirCAS# 159989-64-7 |
- GSK744 (S/GSK1265744)
Catalog No.:BCC3888
CAS No.:1051375-10-0
- Atazanavir
Catalog No.:BCC3622
CAS No.:198904-31-3
- BMS-707035
Catalog No.:BCC2133
CAS No.:729607-74-3
- MK-2048
Catalog No.:BCC2136
CAS No.:869901-69-9
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 159989-64-7 | SDF | Download SDF |
| PubChem ID | 64143 | Appearance | Powder |
| Formula | C32H45N3O4S | M.Wt | 567.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Viracept; AG1341 | ||
| Solubility | DMSO : ≥ 100 mg/mL (176.12 mM) *"≥" means soluble, but saturation unknown. | ||
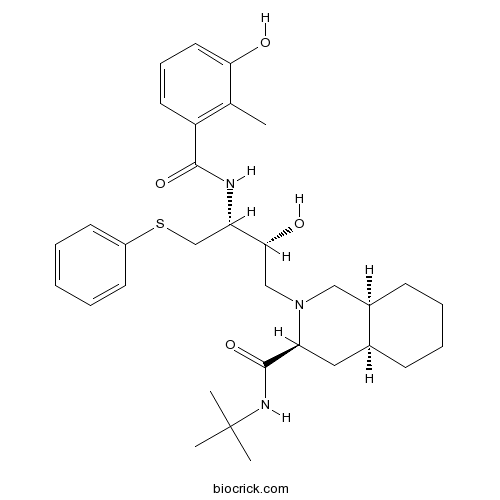
| Chemical Name | (3S,4aS,8aS)-N-tert-butyl-2-[(2R,3R)-2-hydroxy-3-[(3-hydroxy-2-methylbenzoyl)amino]-4-phenylsulfanylbutyl]-3,4,4a,5,6,7,8,8a-octahydro-1H-isoquinoline-3-carboxamide | ||
| SMILES | CC1=C(C=CC=C1O)C(=O)NC(CSC2=CC=CC=C2)C(CN3CC4CCCCC4CC3C(=O)NC(C)(C)C)O | ||
| Standard InChIKey | QAGYKUNXZHXKMR-HKWSIXNMSA-N | ||
| Standard InChI | InChI=1S/C32H45N3O4S/c1-21-25(15-10-16-28(21)36)30(38)33-26(20-40-24-13-6-5-7-14-24)29(37)19-35-18-23-12-9-8-11-22(23)17-27(35)31(39)34-32(2,3)4/h5-7,10,13-16,22-23,26-27,29,36-37H,8-9,11-12,17-20H2,1-4H3,(H,33,38)(H,34,39)/t22-,23+,26-,27-,29+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Nelfinavir(AG-1341) is a potent and orally bioavailable human immunodeficiency virus HIV-1 protease inhibitor (Ki=2 nM) and is widely prescribed in combination with HIV reverse transcriptase inhibitors for the treatment of HIV infection.
IC50 Valur: 2 nM (Ki for HIV-1 protease) [2]
Target: HIV Protease
in vitro: In vitro exposure (72 hours) of HAECs to NEL (0.25-2 μg/mL) decreased both basal (2.5-fold) and insulin-induced NO production (4- to 5-fold). NEL suppressed insulin-induced phosphorylation of both Akt and eNOS at serine residues 473 and 1177, respectively. NEL decreased tyrosine phosphorylation of IR-β, IRS-1, and PI3K. Coexposure to troglitazone (TRO; 250 nM) ameliorated the suppressive effects of NEL on insulin signaling and NO production. Coexposure to TRO also increased eNOS expression in NEL-treated HAECs [1]. AG1343 is a potent enzyme inhibitor (Ki = 2 nM) and antiviral agent (HIV-1 ED50 = 14 nM). An X-ray cocrystal structure of the enzyme-AG1343 complex reveals how the novel thiophenyl ether and phenol-amide substituents of the inhibitor interact with the S1 and S2 subsites of HIV-1 protease, respectively [2].
in vivo: In vivo studies indicate that AG1343 is well absorbed orally in a variety of species and possesses favorable pharmacokinetic properties in humans [2]. References: | |||||

Nelfinavir Dilution Calculator

Nelfinavir Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7612 mL | 8.8062 mL | 17.6125 mL | 35.2249 mL | 44.0311 mL |
| 5 mM | 0.3522 mL | 1.7612 mL | 3.5225 mL | 7.045 mL | 8.8062 mL |
| 10 mM | 0.1761 mL | 0.8806 mL | 1.7612 mL | 3.5225 mL | 4.4031 mL |
| 50 mM | 0.0352 mL | 0.1761 mL | 0.3522 mL | 0.7045 mL | 0.8806 mL |
| 100 mM | 0.0176 mL | 0.0881 mL | 0.1761 mL | 0.3522 mL | 0.4403 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Nelfinavir (brand name Viracept) is an antiretroviral drug used in the treatment of the human immunodeficiency virus (HIV).
- NIBR189
Catalog No.:BCC8056
CAS No.:1599432-08-2
- 3,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6491
CAS No.:159934-13-1
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- Sikokianin C
Catalog No.:BCN6827
CAS No.:159813-69-1
- Ulipristal
Catalog No.:BCC4944
CAS No.:159811-51-5
- Fmoc-Lys(Ac)-OH
Catalog No.:BCC3514
CAS No.:159766-56-0
- Ibutamoren Mesylate
Catalog No.:BCC1638
CAS No.:159752-10-0
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
- Wikstrol A
Catalog No.:BCN7938
CAS No.:159736-35-3
- Saropyrone
Catalog No.:BCN7692
CAS No.:159650-12-1
- 3,4-Secotirucalla-4(28,7,24-triene-3),26-dioic acid
Catalog No.:BCN1549
CAS No.:159623-48-0
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- Antibiotic AB 4063B
Catalog No.:BCN1827
CAS No.:160041-33-8
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
Nelfinavir inhibits proliferation and induces DNA damage in thyroid cancer cells.[Pubmed:28137980]
Endocr Relat Cancer. 2017 Mar;24(3):147-156.
The HIV protease inhibitor Nelfinavir (NFV) inhibits PI3K/AKT and MAPK/ERK signaling pathways, emerging targets in thyroid cancers. We examined the effects of NFV on cancer cells that derived from follicular (FTC), papillary (PTC) and anaplastic (ATC) thyroid cancers. NFV (1-20 microM) was tested in FTC133, BCPAP and SW1736 cell lines. The effects of NFV on cell proliferation were determined in vitro using real-time microscopy and by flow cytometry. DNA damage, apoptotic cell death and expression of molecular markers of epithelial-mesenchymal transition (EMT) were determined by Western blot and real-time PCR. Real-time imaging demonstrated that NFV (10 microM) increased the time required for the cell passage through the phases of cell cycle and induced DNA fragmentation. Growth inhibitory effects of NFV were associated with the accumulation of cells in G0/G1 phase, downregulation of cyclin D1 and cyclin-dependent kinase 4 (CDK4). NFV also induced the expression of gammaH2AX and p53BP1 indicating DNA damage. Treatment with NFV (20 microM) resulted in caspase-3 cleavage in all examined cells. NFV (20 microM) decreased the levels of total and p-AKT in PTEN-deficient FTC133 cells. NFV had no significant effects on total ERK and p-ERK in BRAF-positive BCPAP and SW1736 cells. NFV had no effects on the expression of EMT markers (Twist, Vimentin, E- and N-Cadherin), but inhibited the migration and decreased the abilities of thyroid cancer cells to survive in non-adherent conditions. We conclude that NFV inhibits proliferation and induces DNA damage in thyroid cancer cell lines. Our in vitro data suggest that NFV has a potential to become a new thyroid cancer therapeutic agent.
Nelfinavir and lopinavir impair Trypanosoma cruzi trypomastigote infection in mammalian host cells and show anti-amastigote activity.[Pubmed:27838277]
Int J Antimicrob Agents. 2016 Dec;48(6):703-711.
There is an urgent need to implement new strategies and to search for new chemotherapeutic targets to combat Chagas' disease. In this context, repositioning of clinically approved drugs appears as a viable tool to combat this and several other neglected pathologies. An example is the use of aspartic peptidase inhibitors (PIs) currently applied in human immunodeficiency virus (HIV) treatment against different infectious agents. Therefore, the main objective of this work was to verify the effects of the HIV-PIs Nelfinavir and lopinavir against Trypanosoma cruzi using in vitro models of infection. Cytotoxicity assays with LLC-MK2 epithelial cells and RAW macrophages allowed an evaluation of the effects of HIV-PIs on the interaction between trypomastigotes and these cells as well as the survival of intracellular amastigotes. Pre-treatment of trypomastigotes with Nelfinavir and lopinavir inhibited the association index with LLC-MK2 cells and RAW macrophages in a dose- and time-dependent manner. In addition, Nelfinavir and lopinavir also significantly reduced the number of intracellular amastigotes in both mammalian cell lineages, particularly when administered in daily doses. Both compounds had no effect on nitric oxide production in infected RAW macrophages. These results open the possibility for the use of HIV-PIs as a tangible alternative in the treatment of Chagas' disease. However, the main mechanism of action of Nelfinavir and lopinavir has yet to be elucidated, and more studies using in vivo models must be conducted.
Combining metformin and nelfinavir exhibits synergistic effects against the growth of human cervical cancer cells and xenograft in nude mice.[Pubmed:28252027]
Sci Rep. 2017 Mar 2;7:43373.
Human cervical cancer is the fourth most common carcinoma in women worldwide. However, the emergence of drug resistance calls for continuously developing new anticancer drugs and combination chemotherapy regimens. The present study aimed to investigate the anti-cervical cancer effects of metformin, a first-line therapeutic drug for type 2 diabetes mellitus, and Nelfinavir, an HIV protease inhibitor, when used alone or in combination. We found that both metformin and Nelfinavir, when used alone, were moderately effective in inhibiting proliferation, inducing apoptosis and suppressing migration and invasion of human cervical cell lines HeLa, SiHa and CaSki. When used in combination, these two drugs acted synergistically to inhibit the growth of human cervical cancer cells in vitro and cervical cancer cell xenograft in vivo in nude mice, and suppress cervical cancer cell migration and invasion. The protein expression of phosphoinositide 3-kinase catalytic subunit PI3K(p110alpha), which can promote tumor growth, was remarkably downregulated, while the tumor suppressor proteins p53 and p21 were substantially upregulated following the combinational treatment in vitro and in vivo. These results suggest that clinical use of metformin and Nelfinavir in combination is expected to have synergistic antitumor efficacy and significant potential for the treatment of human cervical cancer.
In-utero exposure to nelfinavir-ethyl methyl sulfone.[Pubmed:27662548]
AIDS. 2016 Nov 13;30(17):2729-2730.
Ethyl methyl sulfone contained in Nelfinavir between 2007 and 2008 accidentally exposed embryos and fetuses to a powerful mutagen. We report data for 101 HIV-uninfected children exposed in utero included in the French prospective national cohort. The incidence of malformation was similar to that in the cohort as a whole with different drug exposures; no children had developed cancer after 9 years of follow-up.


