Antibiotic AB 4063BCAS# 160041-33-8 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160041-33-8 | SDF | Download SDF |
| PubChem ID | 134715053 | Appearance | Powder |
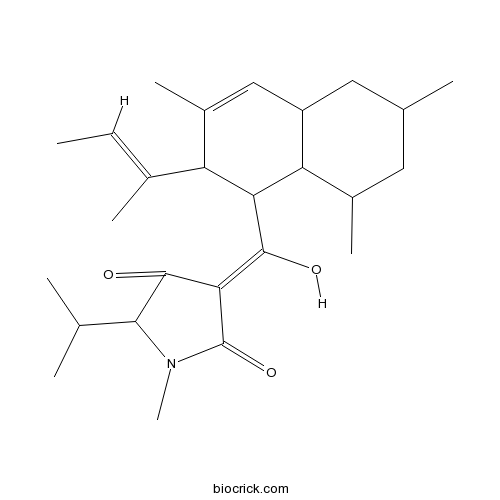
| Formula | C26H39NO3 | M.Wt | 413.6 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3Z)-3-[[2-[(E)-but-2-en-2-yl]-3,6,8-trimethyl-1,2,4a,5,6,7,8,8a-octahydronaphthalen-1-yl]-hydroxymethylidene]-1-methyl-5-propan-2-ylpyrrolidine-2,4-dione | ||
| SMILES | CC=C(C)C1C(C2C(CC(CC2C=C1C)C)C)C(=C3C(=O)C(N(C3=O)C)C(C)C)O | ||
| Standard InChIKey | QWNWSWFYCDLGIB-GHRZEMQSSA-N | ||
| Standard InChI | InChI=1S/C26H39NO3/c1-9-15(5)19-17(7)12-18-11-14(4)10-16(6)20(18)21(19)24(28)22-25(29)23(13(2)3)27(8)26(22)30/h9,12-14,16,18-21,23,28H,10-11H2,1-8H3/b15-9+,24-22- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Antibiotic AB 4063B is active against phytopathogenic fungi. |
| Targets | Antifection |

Antibiotic AB 4063B Dilution Calculator

Antibiotic AB 4063B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4178 mL | 12.089 mL | 24.1779 mL | 48.3559 mL | 60.4449 mL |
| 5 mM | 0.4836 mL | 2.4178 mL | 4.8356 mL | 9.6712 mL | 12.089 mL |
| 10 mM | 0.2418 mL | 1.2089 mL | 2.4178 mL | 4.8356 mL | 6.0445 mL |
| 50 mM | 0.0484 mL | 0.2418 mL | 0.4836 mL | 0.9671 mL | 1.2089 mL |
| 100 mM | 0.0242 mL | 0.1209 mL | 0.2418 mL | 0.4836 mL | 0.6044 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- NIBR189
Catalog No.:BCC8056
CAS No.:1599432-08-2
- 3,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6491
CAS No.:159934-13-1
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- Sikokianin C
Catalog No.:BCN6827
CAS No.:159813-69-1
- Ulipristal
Catalog No.:BCC4944
CAS No.:159811-51-5
- Fmoc-Lys(Ac)-OH
Catalog No.:BCC3514
CAS No.:159766-56-0
- Ibutamoren Mesylate
Catalog No.:BCC1638
CAS No.:159752-10-0
- ISRIB (trans-isomer)
Catalog No.:BCC5340
CAS No.:1597403-47-8
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- SCH 58261
Catalog No.:BCC7306
CAS No.:160098-96-4
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
Electronic structure calculations on the thiazole-containing antibiotic thiostrepton: molecular mechanics, semi-empirical and ab initio analyses.[Pubmed:15713409]
Bioorg Med Chem Lett. 2005 Mar 1;15(5):1471-4.
Thiostrepton is a highly complex cyclic thiazoyl peptide antibiotic and is active against Gram-positive bacteria. Molecular mechanics, semi-empirical and ab initio studies were utilized to further understand the structural and electronic properties of this antibiotic.
Safety evaluation of AB-LIFE((R)) (Lactobacillus plantarum CECT 7527, 7528 and 7529): Antibiotic resistance and 90-day repeated-dose study in rats.[Pubmed:27016492]
Food Chem Toxicol. 2016 Jun;92:117-28.
AB-LIFE((R)) is a probiotic product consisting of equal parts of three strains of Lactobacillus plantarum (CECT 7527, 7528, and 7529) blended with inert excipients. Whole genome sequencing was performed on each of the three strains. Antibiotic resistance was evaluated by genomic mining for resistance genes, and assessment for transferability. No risk of transfer potential was identified for any antibiotic resistance genes in the three strains. AB-LIFE((R)) was evaluated for potential subchronic oral toxicity in rats, with dosages of 300 and 1000 mg/kg BW/day (equivalent to 5.55 x 10(10) and 1.85 x 10(11) CFU/kg BW/day). Survival of the three test strains through the gastrointestinal tract was supported by fecal analysis. No adverse effects were identified with respect to in-life parameters, clinical or anatomic pathology, translocation, or fecal chemical analyses. The no-observed-adverse-effect level (NOAEL) for AB-LIFE((R)) in male and female rats was 1000 mg/kg BW/day (1.85 x 10(11) CFU of AB-LIFE((R))/kg BW/day), the highest dose level evaluated. These results, in conjunction with a previous acute toxicity study in rats, support the conclusion that AB-LIFE((R)) is safe for human consumption.
Studies aimed at the total synthesis of the antitumor antibiotic cochleamycin A. An enantioselective biosynthesis-based pathway to the AB bicyclic core.[Pubmed:11796063]
Org Lett. 2002 Jan 24;4(2):253-6.
[reaction: see text] A convergent, highly enantioselective synthesis of the fully functionalized AB sector of cochleamycin A is described. A pair of building blocks, crafted from L-malic and L-ascorbic acids, are conjoined in a manner that gives rise to an (E,Z,E)-1,6,8-nonatriene. On heating, the latter undergoes stereocontrolled intramolecular Diels-Alder cyclization via an endo transition state.
A cluster randomized trial for the implementation of an antibiotic checklist based on validated quality indicators: the AB-checklist.[Pubmed:25888180]
BMC Infect Dis. 2015 Mar 19;15:134.
BACKGROUND: Recently we developed and validated generic quality indicators that define 'appropriate antibiotic use' in hospitalized adults treated for a (suspected) bacterial infection. Previous studies have shown that with appropriate antibiotic use a reduction of 13% of length of hospital stay can be achieved. Our main objective in this project is to provide hospitals with an antibiotic checklist based on these quality indicators, and to evaluate the introduction of this checklist in terms of (cost-) effectiveness. METHODS/DESIGN: The checklist applies to hospitalized adults with a suspected bacterial infection for whom antibiotic therapy is initiated, at first via the intravenous route. A stepped wedge study design will be used, comparing outcomes before and after introduction of the checklist in nine hospitals in the Netherlands. At least 810 patients will be included in both the control and the intervention group. The primary endpoint is length of hospital stay. Secondary endpoints are appropriate antibiotic use measured by the quality indicators, admission to and duration of intensive care unit stay, readmission within 30 days, mortality, total antibiotic use, and costs associated with implementation and hospital stay. Differences in numerical endpoints between the two periods will be evaluated with mixed linear models; for dichotomous outcomes generalized estimating equation models will be used. A process evaluation will be performed to evaluate the professionals' compliance with use of the checklist. The key question for the economic evaluation is whether the benefits of the checklist, which include reduced antibiotic use, reduced length of stay and associated costs, justify the costs associated with implementation activities as well as daily use of the checklist. DISCUSSION: If (cost-) effective, the AB-checklist will provide physicians with a tool to support appropriate antibiotic use in adult hospitalized patients who start with intravenous antibiotics. TRIAL REGISTRATION: Dutch trial registry: NTR4872.


