SCH 58261Potent, highly selective A2A antagonist CAS# 160098-96-4 |
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- Reparixin L-lysine salt
Catalog No.:BCC1886
CAS No.:266359-93-7
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 160098-96-4 | SDF | Download SDF |
| PubChem ID | 176408 | Appearance | Powder |
| Formula | C18H15N7O | M.Wt | 345.36 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 34 mg/mL (98.45 mM) *"≥" means soluble, but saturation unknown. | ||
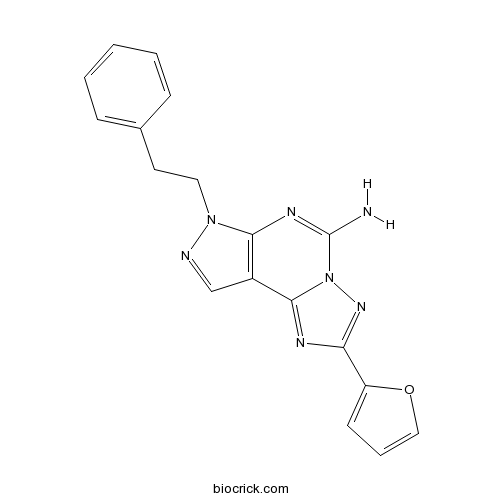
| SMILES | C1=CC=C(C=C1)CCN2C3=C(C=N2)C4=NC(=NN4C(=N3)N)C5=CC=CO5 | ||
| Standard InChIKey | UTLPKQYUXOEJIL-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C18H15N7O/c19-18-22-16-13(11-20-24(16)9-8-12-5-2-1-3-6-12)17-21-15(23-25(17)18)14-7-4-10-26-14/h1-7,10-11H,8-9H2,(H2,19,22) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective A2A adenosine receptor competitive antagonist (Ki = 1.3 nM). Displays 323-, 53- and 100-fold selectivity over A1, A2B and A3 receptors, respectively. |

SCH 58261 Dilution Calculator

SCH 58261 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8955 mL | 14.4776 mL | 28.9553 mL | 57.9106 mL | 72.3882 mL |
| 5 mM | 0.5791 mL | 2.8955 mL | 5.7911 mL | 11.5821 mL | 14.4776 mL |
| 10 mM | 0.2896 mL | 1.4478 mL | 2.8955 mL | 5.7911 mL | 7.2388 mL |
| 50 mM | 0.0579 mL | 0.2896 mL | 0.5791 mL | 1.1582 mL | 1.4478 mL |
| 100 mM | 0.029 mL | 0.1448 mL | 0.2896 mL | 0.5791 mL | 0.7239 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SCH 58261 is the adenosine A2A receptor competitive antagonist. Displays 323-, 53- and 100-fold selectivity over A1, A2B and A3 receptors, respectively. target: adenosine A2A receptor IC50: 15 nM [3] in vitro: NK cells were cultured in NK cell media and preincubated with or without 1 uM SCH58261 30 min before simulation with indicated concentrations of IL-18 (R & D Systems) and IL-12p70 (Australian Biosearch) in the presence or absence of NECA (1 uM) or CGS-21680 (100 nM).[1] in vivo: it was demonstrated that the selective antagonist of the A2Areceptor, SCH58261, administered i.p. starting from the early minutes after ischemia induction, reduces ischemic brain damage and neurological deficit 24 h thereafter. vehicle-rats received saline with Tween 80 (1 %) administered (i.p.) .SCH58261 (0.01 mg/kg, i.p.), administered twice/day for 7 days [2]
References:
[1]. Paul A. Beavis et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A, 2013 Sep 3, 110(36): 14711-14716.
[2]. Alessia Melani et al. Time-course of protection by the selective A2Areceptor antagonist SCH58261 after transient focal cerebral ischemia. Neurological Sciences, August 2015, Volume 36, Issue 8, pp 1441-1448.
[3]. Varani K et al. Pharmacological and biochemical characterization of purified A2a adenosine receptors in human platelet membranes by [3H]-CGS 21680 binding. Br J Pharmacol, 1996 Apr;117(8):1693-701.
- Cryptofolione
Catalog No.:BCN7197
CAS No.:160098-78-2
- Huwentoxin XVI
Catalog No.:BCC8041
CAS No.:1600543-88-1
- Sambutoxin
Catalog No.:BCN1709
CAS No.:160047-56-3
- Antibiotic AB 4063B
Catalog No.:BCN1827
CAS No.:160041-33-8
- Iniparib (BSI-201)
Catalog No.:BCC2208
CAS No.:160003-66-7
- Nelfinavir Mesylate
Catalog No.:BCC1794
CAS No.:159989-65-8
- Nelfinavir
Catalog No.:BCC4138
CAS No.:159989-64-7
- NIBR189
Catalog No.:BCC8056
CAS No.:1599432-08-2
- 3,5-Di-O-caffeoylquinic acid methyl ester
Catalog No.:BCN6491
CAS No.:159934-13-1
- Mc-Val-Cit-PABC-PNP
Catalog No.:BCC4028
CAS No.:159857-81-5
- 4'-O-Methylirenolone
Catalog No.:BCN7174
CAS No.:159853-36-8
- Sikokianin C
Catalog No.:BCN6827
CAS No.:159813-69-1
- 2-Iminopiperidine hydrochloride
Catalog No.:BCC6862
CAS No.:16011-96-4
- L-BMAA hydrochloride
Catalog No.:BCC7400
CAS No.:16012-55-8
- 7-Chloro-1,2,3,4-tetrahydrobenzo[b]azepin-5-one
Catalog No.:BCC8779
CAS No.:160129-45-3
- BIM 23127
Catalog No.:BCC5822
CAS No.:160161-61-5
- SR 11302
Catalog No.:BCC3607
CAS No.:160162-42-5
- 14-Deoxy-11-hydroxyandrographolide
Catalog No.:BCN4702
CAS No.:160242-09-1
- SB 205384
Catalog No.:BCC7095
CAS No.:160296-13-9
- 8-Hydroxybergapten
Catalog No.:BCN2732
CAS No.:1603-47-0
- L-368,899 hydrochloride
Catalog No.:BCC7438
CAS No.:160312-62-9
- Bisdehydroneotuberostemonine
Catalog No.:BCN7072
CAS No.:160333-27-7
- X-NeuNAc
Catalog No.:BCC2063
CAS No.:160369-85-7
- 3',5,5',7-Tetrahydroxyflavanone
Catalog No.:BCN1710
CAS No.:160436-10-2
Biaryl and heteroaryl derivatives of SCH 58261 as potent and selective adenosine A2A receptor antagonists.[Pubmed:18562199]
Bioorg Med Chem Lett. 2008 Jul 15;18(14):4199-203.
SCH 58261 is a reported adenosine A(2A) receptor antagonist, which is active in rat in vivo models of Parkinson's Disease upon ip administration. However, it has poor selectivity versus the A(1) receptor and does not demonstrate oral activity. We report the design and synthesis of biaryl and heteroaryl analogs of SCH 58261 which improve the A(2A) receptor binding selectivity as well as the pharmacokinetic properties of SCH 58261. In particular, the quinoline 25 has excellent A(2A) receptor in vitro binding affinity and selectivity, sustained rat plasma levels upon oral dosing, and is active orally in a rat behavioral assay.
Design, synthesis, and evaluation of fused heterocyclic analogs of SCH 58261 as adenosine A2A receptor antagonists.[Pubmed:18558486]
Bioorg Med Chem Lett. 2008 Jul 15;18(14):4204-9.
SCH 58261 is a reported adenosine A(2A) receptor antagonist which is active in rat in vivo models of Parkinson's Disease upon ip administration. However, it has poor selectivity versus the A(1) receptor and does not demonstrate oral activity. Quinoline analogs have improved upon the selectivity and pharmacokinetics of SCH 58261, but were difficult to handle due to poor aqueous solubility. We report the design and synthesis of fused heterocyclic analogs of SCH 58261 with aqueous solubility as well as improved A(2A) receptor binding selectivity and pharmacokinetic properties. In particular, the tetrahydronaphthyridine 4s has excellent A(2A) receptor in vitro binding affinity and selectivity, is active orally in a rat in vivo model of Parkinson's Disease, and has aqueous solubility of 100 microM at physiological pH.
Behavioral and electrophysiological effects of the adenosine A2A receptor antagonist SCH 58261 in R6/2 Huntington's disease mice.[Pubmed:17720507]
Neurobiol Dis. 2007 Nov;28(2):197-205.
The effect of chronic treatment with the selective adenosine A2A receptor antagonist SCH 58261 on the behavioral and electrophysiological alterations typical of R6/2 mice (a transgenic mouse model of Huntington's disease, HD), has been studied. Starting from 5 weeks of age, R6/2 and wild type (WT) mice were treated daily with SCH 58261 (0.01 mg/kg i.p.) for 7 days. In the following weeks, the ability of mice to perform in the rotarod, plus maze and open field tests were evaluated. In addition, with electrophysiological experiments in corticostriatal slices we tested whether the well-known increased NMDA vulnerability of R6/2 mice was prevented by SCH 58261 treatment. We found that chronic treatment with SCH 58262: i) fully prevented the alterations in emotional/anxious responses displayed by R6/2 mice; ii) did not prevent the impairment in motor coordination; iii) abolished the increase in NMDA-induced toxicity observed in the striatum of HD mice. On balance, targeting A2A receptors seems to have some beneficial effects in HD even though, given the complexity of A2A receptor pharmacology and HD pathogenesis, further studies are necessary to clarify whether A2A receptor antagonists have therapeutic potential in HD.
The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia.[Pubmed:16443200]
Brain Res. 2006 Feb 16;1073-1074:470-80.
We investigated the protective effect of subchronic treatment of the A2A receptor antagonist, SCH 58261 (0.01 mg/kg, i.p.), administered 5 min, 6 h and 15 h after permanent right middle cerebral artery occlusion (MCAo). Twenty-four hours after ischemia, an extensive pallid area, evaluated by cresyl violet staining, is evident in the vascular territories supplied by the MCA, the striatum and the sensory motor cortex. The pallid area reflects the extent of necrotic neurons. Soon after waking, rats showed a definite contralateral turning behavior which was significantly reduced by SCH 58261 treatment. Twenty-four hours after MCAo, SCH 58261 significantly improved the neurological deficit and reduced ischemic damage in the striatum and cortex. Phospho-p38 mitogen-activated protein kinase (MAPK), evaluated by Western Blot, increased by 500% in the ischemic striatum 24 h after MCAo. SCH 58261 treatment significantly reduced phospho-p38 MAPK by 70%. Microglia was immunostained using the OX-42 antibody. Phospho-p38 MAPK and OX-42-immunoreactive cells are localized in the ventral striatum and frontoparietal cortex. Furthermore, both OX-42 and phospho-p38 MAPK-immunoreactive cells have overlapping morphological features, typical of reactive microglia. SCH 58261 reduced phospho-p38 MAPK immunoreactivity in the striatum and in the cortex without changing the microglial cell morphology. These results indicate that the protective effect of the adenosine antagonist SCH 58261 during ischemia is not due to reduced microglial activation but involves inhibition of phospho-p38 MAPK and suggest that treatment with the A2A antagonist from the first hour to several hours after ischemia may be a useful therapeutic approach in cerebral ischemia.
Effects of A(1) and A(2A) adenosine receptor ligands in mouse acute models of pain.[Pubmed:12147316]
Neurosci Lett. 2002 Aug 16;328(3):241-4.
The effects of selective A(1) and A(2A) adenosine receptor compounds in two mouse models of acute nociception were studied: acetic acid-induced writhing and the hot plate assays. Stimulation of A(1) receptors by 2-chloro-N(6)-cyclopentyl-adenosine (CCPA, 0.01-0.1 mg/kg, i.p.; A(1)K(i)=6 nM) or blockade of A(2A) receptors by 5-amino-7-(beta-phenylethyl)-2-(8-furyl)pyrazolo[4,3-e]-1,2,4-triazolo[1,5-c]pyri midine (SCH58261, 1-10 mg/kg, i.p.; A(2)(A)K(i)=1.3 nM) produced anti-nociceptive effects. At the highest dose tested, CCPA and SCH58261 reduced the number of writhings by 79 and 99%, respectively. On the contrary, the A(1) antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (A(1)K(i)=2.8 nM) and the A(2A) agonist 2-(4-[2-carboxyethyl])phenethylamino-5'-N-ethylcarboxamido-adenosine-hydrochlorid e (GGS21680) produced pro-nociceptive effects in both tests. These findings suggest for the first time that blockade of A(2A) adenosine receptors produces anti-nociceptive effects.
The non-xanthine heterocyclic compound SCH 58261 is a new potent and selective A2a adenosine receptor antagonist.[Pubmed:8632302]
J Pharmacol Exp Ther. 1996 Feb;276(2):398-404.
We have characterized the in vitro pharmacological profile of the new potent and selective A2a adenosine receptor antagonist SCH 58261 [7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2, 4-triazolo[1,5-c]pyrimidine]. In binding studies on rat and bovine brain tissues, SCH 58261 showed affinity in the low nanomolar range at A2a adenosine striatal receptors and good A2a adenosine vs. A1 adenosine selectivity (about 50- to 100-fold in rat and bovine brain, respectively). SCH 58261 did not show affinity for either the A3 adenosine receptor or other receptors at concentrations up to 1 microM. Saturation experiments on rat A1 and A2a adenosine receptors indicated the competitive nature of the antagonism. SCH 58261 antagonized competitively the effects induced by the A2a adenosine-selective agonist CGS 21680 (2-[4-(2-carboxyethyl)-phenethyl-amino]-5'-N- ethylcarboxamidoadenosine) in two functional assays, such as inhibition of rabbit platelet aggregation and porcine coronary artery relaxation. Specifically, the compound showed pA2 values of 7.9 and 9.5, respectively. SCH 58261 (300 nM) failed to antagonize 5'-N-ethylcarboxamidoadenosine-induced vasorelaxation in the isolated guinea pig aorta, a response mediated by A2b adenosine receptors. Likewise, at the same concentration, the compound weakly inhibited the A1 adenosine-mediated negative chronotropic effect induced by 2-chloro-N6-cyclopentyladenosine in the isolated rat atria. These data show that SCH 58261 is a potent and selective non-xanthine A2a adenosine antagonist which has competitive properties in biological responses mediated by this receptor subtype. The compound is of interest for investigating the biological role of A2a adenosine receptors and deserves further attention to clarify the therapeutic potential of A2a antagonists.


