Plerixafor (AMD3100)CXCR4 chemokine receptor antagonist CAS# 110078-46-1 |
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- Reparixin L-lysine salt
Catalog No.:BCC1886
CAS No.:266359-93-7
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110078-46-1 | SDF | Download SDF |
| PubChem ID | 65015 | Appearance | Powder |
| Formula | C28H54N8 | M.Wt | 502.78 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMD3100; JM3100 | ||
| Solubility | Ethanol : ≥ 166.66 mg/mL (331.48 mM) DMSO : < 1 mg/mL (insoluble or slightly soluble) DMF : < 1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
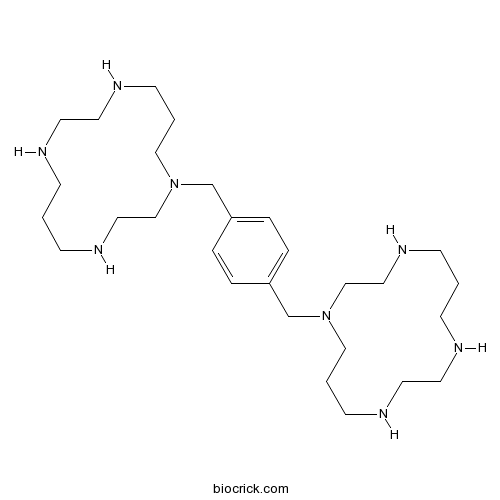
| Chemical Name | 1-[[4-(1,4,8,11-tetrazacyclotetradec-1-ylmethyl)phenyl]methyl]-1,4,8,11-tetrazacyclotetradecane | ||
| SMILES | C1CNCCNCCCN(CCNC1)CC2=CC=C(C=C2)CN3CCCNCCNCCCNCC3 | ||
| Standard InChIKey | YIQPUIGJQJDJOS-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H54N8/c1-9-29-15-17-31-13-3-21-35(23-19-33-11-1)25-27-5-7-28(8-6-27)26-36-22-4-14-32-18-16-30-10-2-12-34-20-24-36/h5-8,29-34H,1-4,9-26H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Plerixafor is a selective CXCR4 antagonist with IC50 of 44 nM.In Vitro:The CXCR4 inhibitor Plerixafor (AMD3100) is a potent inhibitor of CXCL12-mediated chemotaxis (IC50, 5.7 nM) with a potency slightly better than its affinity for CXCR4. Treating the cells with CCX771 or CXCL11 has no effect on CXCL12-mediated MOLT-4 or U937 TEM. In contrast, 10 μM Plerixafor inhibits CXCL12-mediated TEM in both cells lines[1]. Plerixafor (10 μM)-treated cells show a moderate reduction in cell proliferation compared to CXCL12-stimulated cells, which do not reach statistical significance[2].In Vivo:Plerixafor (2 mg/kg) administration to UUO mice exacerbates renal interstitial T cell infiltration, resulting in increased production of the pro-inflammatory cytokines IL-6 and IFN-γ and decreased expression of the anti-inflammatory cytokine IL-10[3]. Both perivascular and interstitial fibrosis are significantly reduced by the CXCR4 antagonist, Plerixafor (AMD3100) at 8 weeks[4]. LD50, mouse, SC: 16.3 mg/kg; LD50, rat, SC: >50 mg/kg; LD50, mouse and rat, IV injection: 5.2 mg/kg. References: | |||||

Plerixafor (AMD3100) Dilution Calculator

Plerixafor (AMD3100) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9889 mL | 9.9447 mL | 19.8894 mL | 39.7788 mL | 49.7235 mL |
| 5 mM | 0.3978 mL | 1.9889 mL | 3.9779 mL | 7.9558 mL | 9.9447 mL |
| 10 mM | 0.1989 mL | 0.9945 mL | 1.9889 mL | 3.9779 mL | 4.9724 mL |
| 50 mM | 0.0398 mL | 0.1989 mL | 0.3978 mL | 0.7956 mL | 0.9945 mL |
| 100 mM | 0.0199 mL | 0.0994 mL | 0.1989 mL | 0.3978 mL | 0.4972 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Plerixafor (AMD3100) is a small-molecule antagonist of CXCR4 and an allosteric agonist of CXCR7 with IC50 of 44 nM and 5.7 nM, respectively [1].
CXCR4 and SDF-1 are key factors in regulating cancer cell invasion and metastasis, and plerixafor can prevent the binding of SDF-1 to CXCR4 for inhibiting cancer metastasis [2]. Plerixafor interfered with CXCL12/CXCR4 mediated retention of hematopoietic stem cells in the bone marrow, and resulted in their mobilization to the blood [3]. Plerixafor amplified the release of circulating neutrophils from the oriented area in the lung, while simultaneously preventing neutrophil returned to the bone marrow [4]. Three adults with WHIM syndrome were subcutaneously injected 0.01 to 0.02 mg/kg plerixafor twice daily for 6 months, circulating leukocytes were constantly increased, and associated with fewer infections [5].
References:
[1]Zabel BA, Wang Y, Lewén S, Berahovich RD, Penfold ME, Zhang P, Powers J, Summers BC, Miao Z, Zhao B, Jalili A, Janowska-Wieczorek A, Jaen JC, Schall TJ. Elucidation of CXCR7-mediated signaling events and inhibition of CXCR4-mediated tumor cell transendothelial migration by CXCR7 ligands. J Immunol. 2009 Sep 1;183(5):3204-11.
[2].Li J, Oupický D. Effect of biodegradability on CXCR4 antagonism, transfection efficacy and antimetastatic activity of polymeric Plerixafor.Biomaterials. 2014 Jul;35(21):5572-9.
[3]. Broxmeyer HE. Chemokines in hematopoiesis. Curr Opin Hematol. 2008 Jan;15(1):49-58.
[4]. Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, Keeble JL, Goh CC, Li JL, Evrard M, Malleret B, Larbi A, Renia L, Haniffa M, Tan SM, Chan JK, Balabanian K, Nagasawa T, Bachelerie F, Hidalgo A, Ginhoux F, Kubes P, Ng LG. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med. 2013 Oct 21;210(11):2321-36.
[5]. McDermott DH, Liu Q, Velez D, Lopez L, Anaya-O'Brien S, Ulrick J, Kwatemaa N, Starling J, Fleisher TA, Priel DA, Merideth MA, Giuntoli RL, Evbuomwan MO, Littel P, Marquesen MM, Hilligoss D, DeCastro R, Grimes GJ, Hwang ST, Pittaluga S, Calvo KR, Stratton P, Cowen EW, Kuhns DB, Malech HL, Murphy PM. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood. 2014 Apr 10;123(15):2308-16.
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
- Succinic acid
Catalog No.:BCN5890
CAS No.:110-15-6
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
- 4-Galloylquinic acid
Catalog No.:BCN3733
CAS No.:110170-37-1
- Ouabain Octahydrate
Catalog No.:BCC5211
CAS No.:11018-89-6
- JZL184
Catalog No.:BCC4790
CAS No.:1101854-58-3
- 1,5,8-Trihydroxy-3-methoxy-2-prenylxanthone
Catalog No.:BCN1623
CAS No.:110187-11-6
- Malonylginsenoside Rb(1)
Catalog No.:BCC9230
CAS No.:88140-34-5
- Cochliophilin A
Catalog No.:BCC8154
CAS No.:110204-45-0
- Ginsenoside Rb2
Catalog No.:BCN1064
CAS No.:11021-13-9
- Ginsenoside Rc
Catalog No.:BCN1072
CAS No.:11021-14-0
Recent advances on the use of the CXCR4 antagonist plerixafor (AMD3100, Mozobil) and potential of other CXCR4 antagonists as stem cell mobilizers.[Pubmed:20826182]
Pharmacol Ther. 2010 Dec;128(3):509-18.
AMD3100 was originally discovered as an anti-HIV agent effective in inhibiting the replication of HIV in vitro at nanomolar concentrations. We found it to be a potent and selective antagonist of CXCR4, the receptor for the chemokine SDF-1 (now called CXCL12). AMD3100 was then developed, and marketed, as a stem cell mobilizer, and renamed plerixafor (Mozobil). The path to the discovery of Mozobil as a stem cell mobilizer was described in Biochem. Pharmacol. 77: 1655-1664 (2009). Here I review the recent advances that have consolidated the role of plerixafor in mobilizing hematopoietic stem cells (HSCs) and hematopoietic progenitor cells (HPCs) from the bone marrow into the blood circulation. Plerixafor acts synergistically with granulocyte colony-stimulating factor (G-CSF), and its usefulness has been proven particularly for the mobilization of HSCs and HPCs for autologous stem cell transplantation in patients with non-Hodgkin's lymphoma (NHL) or multiple myeloma (MM). Plerixafor also has great potential for the treatment of hematological malignancies other than NHL and MM, and non-hematological malignancies, and, eventually, several other diseases depending on the CXCL12-CXCR4 interaction. Various AMD3100 analogs have been described (i.e. AMD11070, AMD3465, KRH-3955, T-140, and 4F-benzoyl-TN14003), primarily as potential anti-HIV agents. They are all strong CXCR4 antagonists. Their role in stem cell mobilization remains to be assessed.
Durable response of glioblastoma to adjuvant therapy consisting of temozolomide and a weekly dose of AMD3100 (plerixafor), a CXCR4 inhibitor, together with lapatinib, metformin and niacinamide.[Pubmed:27489862]
Oncoscience. 2016 Jun 11;3(5-6):156-63.
UNLABELLED: Glioblastoma multiforme (GBM) is a CNS (central nervous system) malignancy with a low cure rate. Median time to progression after standard treatment is 7 months and median overall survival is 15 months [1]. Post-treatment vasculogenesis promoted by recruitment of bone marrow derived cells (BMDCs, CD11b+ myelomonocytes) is one of main mechanisms of GBM resistance to initial chemoradiotherapy treatment [2]. Local secretion of SDF-1, cognate ligand of BMDCs CXCR4 receptors attracts BMDCs to the post-radiation tumor site.[3]. This SDF-1 hypoxia-dependent effect can be blocked by AMD3100 (plerixafor) [4]. We report a GBM case treated after chemo- radiotherapy with plerixafor and a combination of an mTOR, a Sirt1 and an EGFRvIII inhibitor. After one year temozolomide and the EGFRvIII inhibitor were stopped. Plerixafor, and the MTOR and Sirt-1 inhibitors were continued. He is in clinical and radiologic remission 30 months from the initiation of his adjuvant treatment. To our knowledge, this is the first report of a patient treated for over two years with a CXCR4 inhibitor (plerixafor), as part of his adjuvant treatment. We believe there is sufficient experimental evidence to consider AMD3100 (plerixafor) part of the adjuvant treatment of GBM. SIGNIFICANCE: The adjuvant inhibition of GBM vasculogenesis(a process different from local angiogenesis) by specifically blocking the migration of BMDCs to the primary tumor site with inhibitors of the CXCR4/SDF-1 axis represents a potential novel therapeutic approach to GBM. There is significant pre-clinical evidence and validation for its use as demonstrated in a patient derived tumor xenograft model of GBM. Together with other specific anti-tumoral therapies, the active inhibition of vasculogenesis in the adjuvant treatment of GBM is deserving of further exploration.
Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice.[Pubmed:23178377]
Exp Hematol. 2013 Mar;41(3):293-302.e1.
The CXCR4 antagonist Plerixafor (AMD3100) induces the rapid mobilization of hematopoietic stem and progenitor cells into the blood in mice and humans. AMD3100 similarly induces the mobilization of human acute lymphoblastic leukemia (ALL) cells into the blood in mice. In this study, the temporal response of pre-B ALL cells to AMD3100 was compared with that of normal hematopoietic progenitor cells (HPC) using an NOD/SCID xenograft model of ALL and BALB/c mice, respectively. ALL cells remained in the circulation up to 6 hours after AMD3100 administration, by which time normal HPCs were no longer detectable. AMD3100 also increased the proportion of actively cycling ALL cells in the peripheral blood. Together, these data suggest that ALL cells are more sensitive to the effects of bone marrow disruption than normal progenitors. Using the NOD/SCID xenograft model, we demonstrated that AMD3100 increased the efficacy of the cell cycle specific drug vincristine, resulting in reduced disease levels in the blood and spleens of animals over 3 weeks and extended the survival of NOD/SCID mice with ALL. These data demonstrate that mobilizing agents can increase the therapeutic effect of cell cycle dependent chemotherapeutic agents.
Hematopoietic stem cell mobilization with the reversible CXCR4 receptor inhibitor plerixafor (AMD3100)-Polish compassionate use experience.[Pubmed:20938660]
Ann Hematol. 2011 May;90(5):557-68.
Recent developments in the field of targeted therapy have led to the discovery of a new drug, plerixafor, that is a specific inhibitor of the CXCR4 receptor. Plerixafor acts in concert with granulocyte colony-stimulating factor (G-CSF) to increase the number of stem cells circulating in the peripheral blood (PB). Therefore, it has been applied in the field of hematopoietic stem cell mobilization. We analyzed retrospectively data regarding stem cell mobilization with plerixafor in a cohort of 61 patients suffering from multiple myeloma (N = 23), non-Hodgkin's lymphoma (N = 20), or Hodgkin's lymphoma (N = 18). At least one previous mobilization attempt had failed in 83.6% of these patients, whereas 16.4% were predicted to be poor mobilizers. The median number of CD34+ cells in the PB after the first administration of plerixafor was 22/muL (range of 0-121). In total, 85.2% of the patients proceeded to cell collection, and a median of two (range of 0-4) aphereses were performed. A minimum of 2.0 x 10(6) CD34+ cells per kilogram of the patient's body weight (cells/kg b.w.) was collected from 65.6% of patients, and the median number of cells collected was 2.67 x 10(6) CD34+ cells/kg b.w. (0-8.0). Of the patients, 55.7% had already undergone autologous stem cell transplantation, and the median time to neutrophil and platelet reconstitution was 12 and 14 days, respectively. Cases of late graft failure were not observed. We identified the diagnosis of non-Hodgkin's lymphoma and previous radiotherapy as independent factors that contributed to failure of mobilization. The current report demonstrates the satisfactory efficacy of plerixafor plus G-CSF for stem cell mobilization in heavily pre-treated poor or predicted poor mobilizers.


