AMD-070CXCR4 antagonist,potent and selective CAS# 558447-26-0 |
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- Reparixin
Catalog No.:BCC1885
CAS No.:266359-83-5
- Reparixin L-lysine salt
Catalog No.:BCC1886
CAS No.:266359-93-7
- SCH 527123
Catalog No.:BCC1932
CAS No.:473727-83-2
- AMD-070 hydrochloride
Catalog No.:BCC1358
CAS No.:880549-30-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 558447-26-0 | SDF | Download SDF |
| PubChem ID | 11256587 | Appearance | Powder |
| Formula | C21H27N5 | M.Wt | 349.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AMD070,AMD 070 | ||
| Solubility | >17.5mg/mL in DMSO | ||
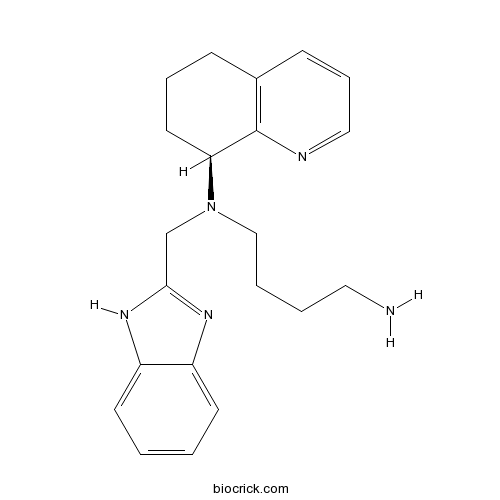
| Chemical Name | N'-(1H-benzimidazol-2-ylmethyl)-N'-[(8S)-5,6,7,8-tetrahydroquinolin-8-yl]butane-1,4-diamine | ||
| SMILES | C1CC(C2=C(C1)C=CC=N2)N(CCCCN)CC3=NC4=CC=CC=C4N3 | ||
| Standard InChIKey | WVLHHLRVNDMIAR-IBGZPJMESA-N | ||
| Standard InChI | InChI=1S/C21H27N5/c22-12-3-4-14-26(15-20-24-17-9-1-2-10-18(17)25-20)19-11-5-7-16-8-6-13-23-21(16)19/h1-2,6,8-10,13,19H,3-5,7,11-12,14-15,22H2,(H,24,25)/t19-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | AMD-070 is a potent and selective antagonist of CXCR4 with an IC50 value of 13 nM in a CXCR4 125I-SDF inhibition binding assay, and inhibits the replication of T-tropic HIV-1 (NL4.3 strain) in MT-4 cells and PBMCs.In Vitro:AMD-070 is confirmed against X4 strain of HIV-1, HIV-1 IIIb in MT-4 cells, and The IC50 values for AMD-070 are 9-fold higher (0.009 μM vs 0.001 μM) and 8.7-fold higher (0.003 μM vs 0.026 μM) in PBMCs compared to MT-4 cells[1]. AMD-070 has antiviral ability with the IC50 value of 15.5 nM[2].In Vivo:AMD-070 (2.5 mg/kg, i.v.) shows promising oral bioavailability in rat and dog. The rate of clearance is species dependent with AMD-070 having lower clearance in dog compared to rat[1]. References: | |||||

AMD-070 Dilution Calculator

AMD-070 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8614 mL | 14.307 mL | 28.6139 mL | 57.2279 mL | 71.5349 mL |
| 5 mM | 0.5723 mL | 2.8614 mL | 5.7228 mL | 11.4456 mL | 14.307 mL |
| 10 mM | 0.2861 mL | 1.4307 mL | 2.8614 mL | 5.7228 mL | 7.1535 mL |
| 50 mM | 0.0572 mL | 0.2861 mL | 0.5723 mL | 1.1446 mL | 1.4307 mL |
| 100 mM | 0.0286 mL | 0.1431 mL | 0.2861 mL | 0.5723 mL | 0.7153 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
AMD-070 is a selective and oral bioavailable antagonist of chemokine receptor CXCR4 with IC50 value of 13 nM [1].
CXCR4 is a G-protein-coupled receptor that plays important roles in tumor development. It affects the migration, proliferation and survival of cancer cells through the CXCL12-mediated MAPK signaling. AMD-070 is an orally bioavailable antagonist of CXCR4 and is found to be an inhibitor of tumor cell migration. CXCR4 is also one of the two chemokine receptors that are used by virus for infecting human cells. As a CXCR4 inhibitor, AMD-070 can repress the replication of X4 (T-tropic) HIV-1 and the interaction of gp120/CXCR4 potently. The mechanistic studies demonstrate that AMD-070 is an allosteric inhibitor. It was found that a hydrogen bond was formed between the benzimidazole of AMD-070 and the Tyr45 residue of CXCR4 whereas the residues Asp262, Asp171 and Glu288 were not involved in the direct interactions with AMD-070 [1, 2 and 3].
AMD-070 is selective against CXCR4 over other related G-protein-coupled chemokine receptors including CXCR1, CXCR2, CCR1, CCR2b, CCR4 and CCR5. The IC50 values of AMD-070 against these GPCRs were all above 10 μM. In HOS cells expressing human CXCR4, AMD-070 inhibited HIV-1 infection with IC50 value of 10 nM. In CD4+CXCR4+T cells, AMD-070 showed anti-HIV-1 activity (IC50 value of 2 nM) through inhibiting the SDF-1 induced calcium flux with IC50 value of 12 nM. In addition, AMD-070 inhibited the competitive binding of 125I-SDF-1with IC50 value of 13 nM. In melanoma cells CHL-1 and A375, treatment of AMD-070 significantly inhibited the migration of cells. Besides that, the void sizes of cells were also increased by the inhibitor treatment [1, 2 and 4].
References:
[1] Skerlj RT, Bridger GJ, Kaller A, McEachern EJ, Crawford JB, Zhou Y, Atsma B, Langille J, Nan S, Veale D, Wilson T, Harwig C, Hatse S, Princen K, De Clercq E, Schols D. Discovery of novel small molecule orally bioavailable C-X-C chemokine receptor 4 antagonists that are potent inhibitors of T-tropic (X4) HIV-1 replication. J Med Chem. 2010 Apr 22;53(8):3376-88.
[2] O'Boyle G, Swidenbank I, Marshall H, Barker CE, Armstrong J, White SA, Fricker SP, Plummer R, Wright M, Lovat PE. Inhibition of CXCR4-CXCL12 chemotaxis in melanoma by AMD11070. Br J Cancer. 2013 Apr 30;108(8):1634-40.
[3] Wong RS, Bodart V, Metz M, Labrecque J, Bridger G, Fricker SP. Comparison of the potential multiple binding modes of bicyclam, monocylam, and noncyclam small-molecule CXC chemokine receptor 4 inhibitors. Mol Pharmacol. 2008 Dec;74(6):1485-95.
[4] Gudmundsson KS, Sebahar PR, Richardson LD, Miller JF, Turner EM, Catalano JG, Spaltenstein A, Lawrence W, Thomson M, Jenkinson S. Amine substituted N-(1H-benzimidazol-2ylmethyl)-5,6,7,8-tetrahydro-8-quinolinamines as CXCR4 antagonists with potent activity against HIV-1. Bioorg Med Chem Lett. 2009 Sep 1;19(17):5048-52.
- Halofuginone
Catalog No.:BCN8519
CAS No.:55837-20-2
- Columbianetin beta-D-glucopyranoside
Catalog No.:BCN8222
CAS No.:55836-35-6
- Betahistine 2HCl
Catalog No.:BCC4525
CAS No.:5579-84-0
- Boc-D-Allo-Ile-OH
Catalog No.:BCC2603
CAS No.:55780-90-0
- Sanguinarine chloride
Catalog No.:BCC6481
CAS No.:5578-73-4
- Sunitinib
Catalog No.:BCC4064
CAS No.:557795-19-4
- WZ811
Catalog No.:BCC4448
CAS No.:55778-02-4
- Crocin II
Catalog No.:BCN1027
CAS No.:55750-84-0
- Litorin
Catalog No.:BCC5846
CAS No.:55749-97-8
- Oxoglaucine
Catalog No.:BCN2650
CAS No.:5574-24-3
- 3-Deoxo-1Beta-methoxyjioglutolide
Catalog No.:BCN7034
CAS No.:55732-36-0
- Stigmasta-4,22-dien-3-one
Catalog No.:BCN5745
CAS No.:55722-32-2
- 1-Methoxymethyl-beta-carboline
Catalog No.:BCN7911
CAS No.:55854-60-9
- VER-49009
Catalog No.:BCC5297
CAS No.:558640-51-0
- 6-beta-Hydroxyhyoscyamine
Catalog No.:BCN1915
CAS No.:55869-99-3
- Boc-D-Lys(Z)-OH
Catalog No.:BCC3423
CAS No.:55878-47-2
- 6-Hydroxysugiol
Catalog No.:BCN3154
CAS No.:55898-07-2
- Morolic acid
Catalog No.:BCN7475
CAS No.:559-68-2
- beta-Amyrin
Catalog No.:BCN5746
CAS No.:559-70-6
- Friedelin
Catalog No.:BCN5747
CAS No.:559-74-0
- Cucurbitacin R
Catalog No.:BCN7877
CAS No.:55903-92-9
- Polyphyllin VI
Catalog No.:BCN1053
CAS No.:55916-51-3
- Pennogenin 3-O-beta-chacotrioside
Catalog No.:BCN6707
CAS No.:55916-52-4
- Betamethasone 17,21-dipropionate
Catalog No.:BCC8875
CAS No.:5593-20-4
Impact of a CXCL12/CXCR4 Antagonist in Bleomycin (BLM) Induced Pulmonary Fibrosis and Carbon Tetrachloride (CCl4) Induced Hepatic Fibrosis in Mice.[Pubmed:26998906]
PLoS One. 2016 Mar 21;11(3):e0151765.
Modulation of chemokine CXCL12 and its receptor CXCR4 has been implicated in attenuation of bleomycin (BLM)-induced pulmonary fibrosis and carbon tetrachloride (CCl4)-induced hepatic injury. In pulmonary fibrosis, published reports suggest that collagen production in the injured lung is derived from fibrocytes recruited from the circulation in response to release of pulmonary CXCL12. Conversely, in hepatic fibrosis, resident hepatic stellate cells (HSC), the key cell type in progression of fibrosis, upregulate CXCR4 expression in response to activation. Further, CXCL12 induces HSC proliferation and subsequent production of collagen I. In the current study, we evaluated AMD070, an orally bioavailable inhibitor of CXCL12/CXCR4 in alleviating BLM-induced pulmonary and CCl4-induced hepatic fibrosis in mice. Similar to other CXCR4 antagonists, treatment with AMD070 significantly increased leukocyte mobilization. However, in these two models of fibrosis, AMD070 had a negligible impact on extracellular matrix deposition. Interestingly, our results indicated that CXCL12/CXCR4 signaling has a role in improving mortality associated with BLM induced pulmonary injury, likely through dampening an early inflammatory response and/or vascular leakage. Together, these findings indicate that the CXCL12-CXCR4 signaling axis is not an effective target for reducing fibrosis.
New targets in antiretroviral therapy 2006.[Pubmed:19372844]
Curr Opin HIV AIDS. 2006 Sep;1(5):437-41.
PURPOSE OF REVIEW: Novel antiretroviral therapies that utilize new viral and cellular targets are needed to increase the range of treatment options for individuals living with HIV. Several novel therapies are now proceeding through clinical trials, and clinical and safety information is starting to accumulate. RECENT FINDINGS: The review outlines progress to date and important considerations for the drugs that are furthest along in clinical development from the following new classes of anti-HIV therapy: co-receptor inhibitors (maraviroc, vicriviroc and AMD-070), integrase inhibitors (MK-0518 and GS-9137), maturation inhibitors (PA-457), and entry inhibitors (TNX-355). The focus is on novel drugs for which initial data are available from studies in both healthy volunteers and studies involving HIV-infected participants. SUMMARY: Although the drugs reviewed may potentially provide important new options for existing antiretroviral treatment regimens, much information regarding the safety, efficacy and positioning of the drugs remains to be determined. As further clinical trial information accumulates, it will be important for HIV clinicians to monitor the progress of these novel therapies.
Gateways to clinical trials.[Pubmed:15538546]
Methods Find Exp Clin Pharmacol. 2004 Sep;26(7):587-612.
Gateways to Clinical Trials is a guide to the most recent clinical trials in current literature and congresses. The data in the following tables has been retrieved from the Clinical Trials Knowledge Area of Prous Science Integrity, the drug discovery and development portal, http://integrity.prous.com. This issue focuses on the following selection of drugs: 101M, 166Ho-DOTMP, 3-AP; Abatacept, abetimus sodium, ACR-16, adefovir dipivoxil, alefacept, AMD-070, aminolevulinic acid hexyl ester, anatumomab mafenatox, anti-CTLA-4 MAb, antigastrin therapeutic vaccine, AP-12009, AP-23573, APC-8024, aripiprazole, ATL-962, atomoxetine hydrochloride; Bevacizumab, bimatoprost, bortezomib, bosentan, BR-1; Calcipotriol/betamethasone dipropionate, cinacalcet hydrochloride, clofazimine, colchicine, cold-adapted influenza vaccine trivalent, CRM197; Desloratadine, desoxyepothilone B, diethylhomospermine; Edodekin alfa, efalizumab, elcometrine, eletriptan, enfuvirtide, entecavir, EP-2101, eplerenone, erlotinib hydrochloride, etoricoxib, everolimus, exherin, ezetimibe; Febuxostat, fluorescein lisicol, fosamprenavir calcium, frovatriptan; Hemoglobin raffimer, HSPPC-96, human insulin; Imatinib mesylate, insulin detemir, insulin glargine, IRX-2, istradefylline, IV gamma-globulin, ixabepilone; Kahalalide F; L-759274, levodopa/carbidopa/entacapone, licofelone, lonafarnib, lopinavir, lurtotecan, LY-156735; MAb G250, mecasermin, melatonin, midostaurin, muraglitazar; Nesiritide, nitronaproxen; O6-Benzylguanine, olmesartan medoxomil, olmesartan medoxomil/hydrochlorothiazide, omapatrilat, oral insulin; Parecoxib sodium, PCK-3145, peginterferon alfa-2a, peginterferon alfa-2b, peginterferon alfa-2b/ ribavirin, pemetrexed disodium, peptide YY3-36, PG-CPT, phenoxodiol, pimecrolimus, posaconazole; Rasagiline mesilate, rDNA insulin, RG228, rimonabant hydrochloride, rosuvastatin calcium, rotigotine hydrochloride; S-3304, safinamide mesilate, salcaprozic acid sodium salt, SDZ-SID-791, SGN-30, soblidotin, squalamine; Telmisartan/hydrochlorothiazide, testosterone gel, TF(c)-KLH conjugate vaccine, TH-9507, theraloc, tipifarnib, tocilizumab, travoprost; ValboroPro, valdecoxib, veglin, voriconazole; Ximelagatran.


