Stigmasta-4,22-dien-3-oneCAS# 55722-32-2 |
Quality Control & MSDS
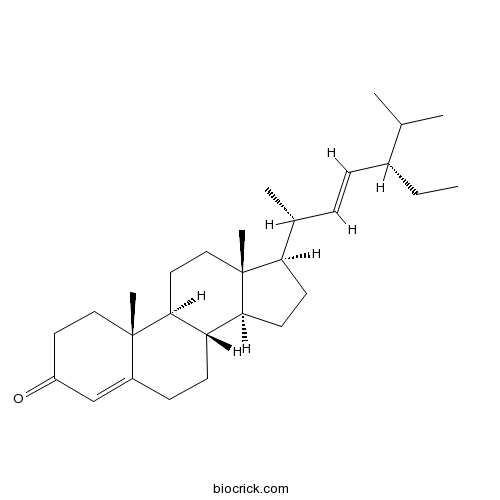
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55722-32-2 | SDF | Download SDF |
| PubChem ID | 6442194 | Appearance | Powder |
| Formula | C29H46O | M.Wt | 410.7 |
| Type of Compound | Steroids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (8S,9S,10R,13R,14S,17R)-17-[(E,2R,5S)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-1,2,6,7,8,9,11,12,14,15,16,17-dodecahydrocyclopenta[a]phenanthren-3-one | ||
| SMILES | CCC(C=CC(C)C1CCC2C1(CCC3C2CCC4=CC(=O)CCC34C)C)C(C)C | ||
| Standard InChIKey | MKGZDUKUQPPHFM-LPJPOILFSA-N | ||
| Standard InChI | InChI=1S/C29H46O/c1-7-21(19(2)3)9-8-20(4)25-12-13-26-24-11-10-22-18-23(30)14-16-28(22,5)27(24)15-17-29(25,26)6/h8-9,18-21,24-27H,7,10-17H2,1-6H3/b9-8+/t20-,21-,24+,25-,26+,27+,28+,29-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Stigmasta-4,22-dien-3-one has antioxidant activity. |

Stigmasta-4,22-dien-3-one Dilution Calculator

Stigmasta-4,22-dien-3-one Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4349 mL | 12.1743 mL | 24.3487 mL | 48.6973 mL | 60.8717 mL |
| 5 mM | 0.487 mL | 2.4349 mL | 4.8697 mL | 9.7395 mL | 12.1743 mL |
| 10 mM | 0.2435 mL | 1.2174 mL | 2.4349 mL | 4.8697 mL | 6.0872 mL |
| 50 mM | 0.0487 mL | 0.2435 mL | 0.487 mL | 0.9739 mL | 1.2174 mL |
| 100 mM | 0.0243 mL | 0.1217 mL | 0.2435 mL | 0.487 mL | 0.6087 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Boc-D-Ile-OH
Catalog No.:BCC3407
CAS No.:55721-65-8
- Salinomycin sodium salt
Catalog No.:BCC1917
CAS No.:55721-31-8
- Secalciferol
Catalog No.:BCC1942
CAS No.:55721-11-4
- (24S)-24,25-Dihydroxyvitamin D3
Catalog No.:BCC1290
CAS No.:55700-58-8
- 1-Octacosanol
Catalog No.:BCN2973
CAS No.:557-61-9
- TCN 213
Catalog No.:BCC6123
CAS No.:556803-08-8
- Boc-D-Thr-OH
Catalog No.:BCC3453
CAS No.:55674-67-4
- Deltonin
Catalog No.:BCC8933
CAS No.:55659-75-1
- Syneilesine
Catalog No.:BCN2144
CAS No.:55652-65-8
- Cepharadione B
Catalog No.:BCN6524
CAS No.:55610-02-1
- Cepharadione A
Catalog No.:BCN3950
CAS No.:55610-01-0
- Aristololactam II
Catalog No.:BCN8095
CAS No.:55610-00-9
- 3-Deoxo-1Beta-methoxyjioglutolide
Catalog No.:BCN7034
CAS No.:55732-36-0
- Oxoglaucine
Catalog No.:BCN2650
CAS No.:5574-24-3
- Litorin
Catalog No.:BCC5846
CAS No.:55749-97-8
- Crocin II
Catalog No.:BCN1027
CAS No.:55750-84-0
- WZ811
Catalog No.:BCC4448
CAS No.:55778-02-4
- Sunitinib
Catalog No.:BCC4064
CAS No.:557795-19-4
- Sanguinarine chloride
Catalog No.:BCC6481
CAS No.:5578-73-4
- Boc-D-Allo-Ile-OH
Catalog No.:BCC2603
CAS No.:55780-90-0
- Betahistine 2HCl
Catalog No.:BCC4525
CAS No.:5579-84-0
- Columbianetin beta-D-glucopyranoside
Catalog No.:BCN8222
CAS No.:55836-35-6
- Halofuginone
Catalog No.:BCN8519
CAS No.:55837-20-2
- AMD-070
Catalog No.:BCC1357
CAS No.:558447-26-0
Anti-platelet aggregation alkaloids and lignans from Hernandia nymphaeifolia.[Pubmed:10821052]
Planta Med. 2000 Apr;66(3):251-6.
A new aporphine, N-(N-methylcarbamoyl)-O-methyl-bulbocapnine (1), together with seven known compounds, (-)-5'-methoxypodorhizol (2), a mixture of beta-sitosterone (3) and Stigmasta-4,22-dien-3-one (4), a mixture of 3 beta-hydroxystigmast-5-en-7-one (5) and 3 beta-hydroxystigmasta-5,22-dien-7-one (6), and a mixture of 6 alpha-hydroxystigmast-4-en-3-one (7) and 6 alpha-hydroxyStigmasta-4,22-dien-3-one (8), were isolated in continuing studies on the trunk bark of Formosan Hernandia nymphaeifolia. The structures of these compounds were determined through spectral analyses. In addition, the previously reported six alkaloids, laurotetanine, oxohernagine, thalicarpine, reticuline, (+)-vateamine-2'-beta-N-oxide, (+)-hernandaline and six lignans, (+)-epiaschantin, (+)-epimagnolin, (+)-epiyangambin, (-)-hernone, (-)-yatein, (-)-deoxypodophyllotoxin were demonstrated to have anti-platelet aggregation activity.
Antioxidant and anticancer aporphine alkaloids from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena.[Pubmed:25372397]
Molecules. 2014 Nov 3;19(11):17829-38.
Fifteen compounds were extracted and purified from the leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena. These compounds include liriodenine (1), lysicamine (2), (-)-anonaine (3), (-)-asimilobine (4), (-)-caaverine (5), (-)-N-methylasimilobine (6), (-)-nuciferine (7), (-)-nornuciferine (8), (-)-roemerine (9), 7-hydroxydehydronuciferine (10) cepharadione B (11), beta-sitostenone (12), Stigmasta-4,22-dien-3-one (13) and two chlorophylls: pheophytin-a (14) and aristophyll-C (15). The anti-oxidation activity of the compounds was examined by antiradical scavenging, metal chelating and ferric reducing power assays. The results have shown that these compounds have antioxidative activity. The study has also examined the antiproliferation activity of the isolated compounds against human melanoma, prostate and gastric cancer cells. The results shown that 7-hydroxydehydronuciferine (10) significantly inhibited the proliferation of melanoma, prostate and gastric cancer cells. Together, these findings suggest that leaves of Nelumbo nucifera Gaertn. cv. Rosa-plena are a good resource for obtaining the biologically active substances with antioxidant properties.
Quantitative Structure Inter-Activity Relationship (QSInAR). Cytotoxicity Study of Some Hemisynthetic and Isolated Natural Steroids and Precursors on Human Fibrosarcoma Cells HT1080.[Pubmed:25134765]
Molecules. 2011 Aug 5;16(8):6603-20.
Combined experimental and quantitative structure inter-activity relationship (QSIAR) computation methods were advanced in order to establish the structural and mechanistic influences that steroids and triterpenes, either as newly synthesized or naturally isolated products, have on human HT1080 mammalian cancer cells. The main Hansch structural indicators such as hydrophobicity (LogP), polarizability (POL) and total energy (Etot) were considered and both the structure-projected as well as globally computed correlations were reported; while the inter-activity correlation of the global activity with those projected on structural information was revealed as equal to the direct structural-activity one for the trial sets of compounds, the prediction for the testing set of molecules reported even superior performances respecting those characteristic for the calibration set, validating therefore the present QSInAR models; accordingly, it follows that the LogP carries the most part of the cytotoxic signal, while POL has little influence on inhibiting tumor growth-A complementary behavior with their earlier known influence on genotoxic carcinogenesis. Regarding the newly hemisynthetic compounds it was found that Stigmasta-4,22-dien-3-one is not adapted for cell membrane diffusion; it is recommended that aminocinnamyl chlorohydrate be further modified in order to acquire better steric influence, while aminocinnamyl-2,3,4,6-O-tetraacetyl-alpha-D-glucopyranoside was identified as being inhibited in the tumor cell by other molecular mechanisms-here not revealed-although it has a moderate-high anti-cancer structurally predicted activity.


