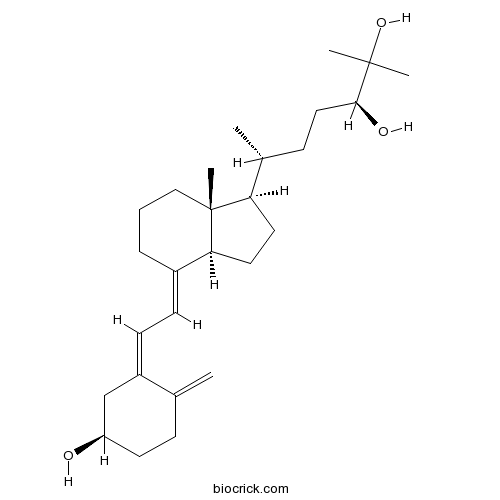
(24S)-24,25-Dihydroxyvitamin D3Inactive form of vitamin D3 CAS# 55700-58-8 |
- 3-O-(2-Aminoethyl)-25-hydroxyvitamin D3
Catalog No.:BCC1309
CAS No.:163018-26-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 55700-58-8 | SDF | Download SDF |
| PubChem ID | 6439679 | Appearance | Powder |
| Formula | C27H44O3 | M.Wt | 416.64 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | (24S)-24,25-Dihydroxycholecalciferol | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (3S,6R)-6-[(1R,3aS,4E,7aR)-4-[(2Z)-2-[(5R)-5-hydroxy-2-methylidenecyclohexylidene]ethylidene]-7a-methyl-2,3,3a,5,6,7-hexahydro-1H-inden-1-yl]-2-methylheptane-2,3-diol | ||
| SMILES | CC(CCC(C(C)(C)O)O)C1CCC2C1(CCCC2=CC=C3CC(CCC3=C)O)C | ||
| Standard InChIKey | FCKJYANJHNLEEP-XUCUACLUSA-N | ||
| Standard InChI | InChI=1S/C27H44O3/c1-18-8-12-22(28)17-21(18)11-10-20-7-6-16-27(5)23(13-14-24(20)27)19(2)9-15-25(29)26(3,4)30/h10-11,19,22-25,28-30H,1,6-9,12-17H2,2-5H3/b20-10+,21-11-/t19-,22-,23-,24+,25+,27-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (24S)-24,25-Dihydroxycholecalciferol is an inactive form of vitamin D3 which undergoes various levels of hydroxylation to form active vitamin D3 analogs.
IC50 value:
Target: Vitamin D3 analog
1α-Hydroxyvitamin D3 (alfacalcidol) is a synthetic analog that is metabolized to 1,25-dihydroxycholecalciferol, the biologically active form of vitamin D3. Other analogues of cholecalciferol result from different hydroxylations. 24S,25-Dihydroxyvitamin D3 should not be confused with 24R,25-Dihydroxyvitamin D3. References: | |||||

(24S)-24,25-Dihydroxyvitamin D3 Dilution Calculator

(24S)-24,25-Dihydroxyvitamin D3 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4002 mL | 12.0008 mL | 24.0015 mL | 48.0031 mL | 60.0038 mL |
| 5 mM | 0.48 mL | 2.4002 mL | 4.8003 mL | 9.6006 mL | 12.0008 mL |
| 10 mM | 0.24 mL | 1.2001 mL | 2.4002 mL | 4.8003 mL | 6.0004 mL |
| 50 mM | 0.048 mL | 0.24 mL | 0.48 mL | 0.9601 mL | 1.2001 mL |
| 100 mM | 0.024 mL | 0.12 mL | 0.24 mL | 0.48 mL | 0.6 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cholecalciferol is an inactive form of vitamin D3 which undergoes various levels of hydroxylation to form active vitamin D3 analogs. 1α-Hydroxyvitamin D3 (alfacalcidol) is a synthetic analog that is metabolized to 1,25-dihydroxycholecalciferol, the biologically active form of vitamin D3. Other analogues of cholecalciferol result from different hydroxylations. 24S,25-Dihydroxyvitamin D3 should not be confused with 24R,25-Dihydroxyvitamin D3.
- 1-Octacosanol
Catalog No.:BCN2973
CAS No.:557-61-9
- TCN 213
Catalog No.:BCC6123
CAS No.:556803-08-8
- Boc-D-Thr-OH
Catalog No.:BCC3453
CAS No.:55674-67-4
- Deltonin
Catalog No.:BCC8933
CAS No.:55659-75-1
- Syneilesine
Catalog No.:BCN2144
CAS No.:55652-65-8
- Cepharadione B
Catalog No.:BCN6524
CAS No.:55610-02-1
- Cepharadione A
Catalog No.:BCN3950
CAS No.:55610-01-0
- Aristololactam II
Catalog No.:BCN8095
CAS No.:55610-00-9
- 1-Oxo-4-hydroxy-2-en-4-ethylcyclohexa-5,8-olide
Catalog No.:BCN1417
CAS No.:55604-88-1
- Alverine Citrate
Catalog No.:BCC4619
CAS No.:5560-59-8
- Alliin
Catalog No.:BCN3869
CAS No.:556-27-4
- H-D-Tyr-OH
Catalog No.:BCC3134
CAS No.:556-02-5
- Secalciferol
Catalog No.:BCC1942
CAS No.:55721-11-4
- Salinomycin sodium salt
Catalog No.:BCC1917
CAS No.:55721-31-8
- Boc-D-Ile-OH
Catalog No.:BCC3407
CAS No.:55721-65-8
- Stigmasta-4,22-dien-3-one
Catalog No.:BCN5745
CAS No.:55722-32-2
- 3-Deoxo-1Beta-methoxyjioglutolide
Catalog No.:BCN7034
CAS No.:55732-36-0
- Oxoglaucine
Catalog No.:BCN2650
CAS No.:5574-24-3
- Litorin
Catalog No.:BCC5846
CAS No.:55749-97-8
- Crocin II
Catalog No.:BCN1027
CAS No.:55750-84-0
- WZ811
Catalog No.:BCC4448
CAS No.:55778-02-4
- Sunitinib
Catalog No.:BCC4064
CAS No.:557795-19-4
- Sanguinarine chloride
Catalog No.:BCC6481
CAS No.:5578-73-4
- Boc-D-Allo-Ile-OH
Catalog No.:BCC2603
CAS No.:55780-90-0
Naturally occurring 24,25-dihydroxyvitamin D3 is a mixture of both C-24R and C-24S epimers.[Pubmed:6091567]
Arch Biochem Biophys. 1984 Oct;234(1):97-104.
Tritium-labeled 24,25-dihydroxyvitamin D3 was prepared both in vitro, by using chick kidney homogenates, and in vivo in rats from [26,27-methyl-3H]25-hydroxyvitamin D3. These compounds were mixed with synthetic 24(R),25- and 24(S),25-dihydroxyvitamin D3, converted to the corresponding trimethylsilyl ether derivatives, and analyzed by a high-pressure liquid chromatography procedure that separates the derivatized isomers. The tritium-labeled 24,25-dihydroxyvitamin D3 derivatives were found to be a mixture of both the 24(R) and 24(S) epimers; the ratio was found to be 96.4:3.6 in chick kidney homogenates and 96.8:3.2 in the serum of rats under physiological conditions. In addition, nonradioactive 24,25-dihydroxyvitamin D3 isolated from the serum of rats given large doses of vitamin D3 was shown to be an 89.5:10.5 mixture of the 24(R) and 24(S) isomers. When 25-hydroxy-24-oxo-vitamin D3 was utilized as a substrate, it was found to be more selectively reduced to 24(S),25-dihydroxyvitamin D3 than 24(R),25-dihydroxyvitamin D3 by the renal enzyme. The 24(S),25-dihydroxyvitamin D3 has been identified by ultraviolet absorption spectrophotometry, cochromatography with an authentic standard, and mass spectrometry. The reduced metabolites of 25-hydroxy-24-oxo-vitamin D3 were a 1:50 mixture of the 24(R) and 24(S) epimers. There are two known metabolic pathways leading to 24,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3; one is 24(R)-hydroxylation of 25-hydroxyvitamin D3 and the other is reduction of 25-hydroxy-24-oxo-vitamin D3. In contrast, 24(S),25-dihydroxyvitamin D3 is produced only by reduction of 25-hydroxy-24-oxo-vitamin D3 in the kidney. Therefore, naturally occurring 24,25-dihydroxyvitamin D3 is a mixture of the 24(R) and 24(S) isomers, and not just the 24(R) isomer as reported previously.


