Sorbic acidCAS# 110-44-1 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110-44-1 | SDF | Download SDF |
| PubChem ID | 643460 | Appearance | White powder |
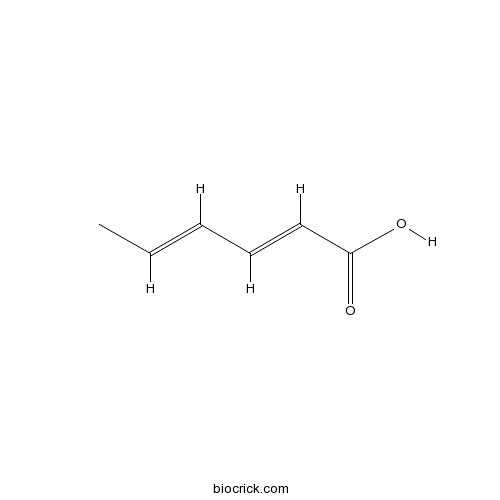
| Formula | C6H8O2 | M.Wt | 112.13 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | 2E,4E-Hexadienoic acid | ||
| Solubility | Freely soluble in ethanol; slightly soluble in water | ||
| Chemical Name | (2E,4E)-hexa-2,4-dienoic acid | ||
| SMILES | CC=CC=CC(=O)O | ||
| Standard InChIKey | WSWCOQWTEOXDQX-MQQKCMAXSA-N | ||
| Standard InChI | InChI=1S/C6H8O2/c1-2-3-4-5-6(7)8/h2-5H,1H3,(H,7,8)/b3-2+,5-4+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sorbic acid is important food preservatives and powerful fungistatic agents, it inhibits various bacteria, including sporeformers, at various stages of their life cycle (germination, outgrowth and cell division). Sorbic acid is a more potent uncoupler of the membrane potential than acetic acid, the effect may also slow the rate of ATP synthesis significantly and may thus (partially) explain Sorbic acid's effectiveness. |
| Targets | Antifection | ATP | MAPK |
| In vitro | Distinct effects of sorbic acid and acetic acid on the electrophysiology and metabolism of Bacillus subtilis.[Pubmed: 25038097]Appl Environ Microbiol. 2014 Oct;80(19):5918-26.Sorbic acid and acetic acid are among the weak organic acid preservatives most commonly used to improve the microbiological stability of foods. They have similar pKa values, but Sorbic acid is a far more potent preservative. Weak organic acids are most effective at low pH. Under these circumstances, they are assumed to diffuse across the membrane as neutral undissociated acids.
Impact of sorbic acid on germination and outgrowth heterogeneity of Bacillus cereus ATCC 14579 spores.[Pubmed: 23001664]Appl Environ Microbiol. 2012 Dec;78(23):8477-80.Population heterogeneity complicates the predictability of the outgrowth kinetics of individual spores.
Effect of sorbic acid on the storage quality of Kaladhi -an acid coagulated milk product[Pubmed: 25477678]J Food Sci Technol. 2014 Dec;51(12):4040-6.The present study was conducted to determine the effect of three different levels of Sorbic acid (0.1 %, 0.2 % and 0.3 %) on the shelf life and storage quality of Kaladhi.
|
| Kinase Assay | Sorbic acid stress activates the Candida glabrata high osmolarity glycerol MAP kinase pathway.[Pubmed: 24324463]Front Microbiol. 2013 Nov 26;4:350.Weak organic acids such as Sorbic acid are important food preservatives and powerful fungistatic agents. |

Sorbic acid Dilution Calculator

Sorbic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.9182 mL | 44.5911 mL | 89.1822 mL | 178.3644 mL | 222.9555 mL |
| 5 mM | 1.7836 mL | 8.9182 mL | 17.8364 mL | 35.6729 mL | 44.5911 mL |
| 10 mM | 0.8918 mL | 4.4591 mL | 8.9182 mL | 17.8364 mL | 22.2955 mL |
| 50 mM | 0.1784 mL | 0.8918 mL | 1.7836 mL | 3.5673 mL | 4.4591 mL |
| 100 mM | 0.0892 mL | 0.4459 mL | 0.8918 mL | 1.7836 mL | 2.2296 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
- Succinic acid
Catalog No.:BCN5890
CAS No.:110-15-6
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- Kifunensine
Catalog No.:BCC7601
CAS No.:109944-15-2
- Sodium prasterone sulfate
Catalog No.:BCC9149
CAS No.:1099-87-2
- Granisetron
Catalog No.:BCC1601
CAS No.:109889-09-0
- CGS 9343B
Catalog No.:BCC7303
CAS No.:109826-27-9
- Kaempferol 5,7,4'-trimethyl ether
Catalog No.:BCN6587
CAS No.:1098-92-6
- Harmalidine
Catalog No.:BCN5889
CAS No.:109794-97-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
- Methyl hesperidin
Catalog No.:BCN6341
CAS No.:11013-97-1
Distinct effects of sorbic acid and acetic acid on the electrophysiology and metabolism of Bacillus subtilis.[Pubmed:25038097]
Appl Environ Microbiol. 2014 Oct;80(19):5918-26.
Sorbic acid and acetic acid are among the weak organic acid preservatives most commonly used to improve the microbiological stability of foods. They have similar pKa values, but Sorbic acid is a far more potent preservative. Weak organic acids are most effective at low pH. Under these circumstances, they are assumed to diffuse across the membrane as neutral undissociated acids. We show here that the level of initial intracellular acidification depends on the concentration of undissociated acid and less on the nature of the acid. Recovery of the internal pH depends on the presence of an energy source, but acidification of the cytosol causes a decrease in glucose flux. Furthermore, Sorbic acid is a more potent uncoupler of the membrane potential than acetic acid. Together these effects may also slow the rate of ATP synthesis significantly and may thus (partially) explain Sorbic acid's effectiveness.
"Effect of sorbic acid on the storage quality of Kaladhi-an acid coagulated milk product".[Pubmed:25477678]
J Food Sci Technol. 2014 Dec;51(12):4040-6.
The present study was conducted to determine the effect of three different levels of Sorbic acid (0.1 %, 0.2 % and 0.3 %) on the shelf life and storage quality of Kaladhi. Kaladhi was prepared from pasteurized buffalo milk standardized to 6 % fat and 9 % SNF with coagulation at 40 degrees C using 5 % lactic acid as a coagulant. Kaladhi prepared without Sorbic acid served as control and was compared with the products treated with different levels of Sorbic acid for a storage period of 35 days at ambient temperature. The results showed a significant (P < 0.05) effect of Sorbic acid on most of the physicochemical parameters i.e. titratable acidity, free fatty acid content (% oleic acid) and thiobarbituric acid value which showed a decreasing trend with increasing concentration of Sorbic acid. However, a non-significant (P > 0.05) effect of Sorbic acid was observed on pH and proximate parameters of the product. Kaladhi treated with 0.3 % Sorbic acid retained most desirable physicochemical and sensory properties throughout the storage period hence, was considered the best.
Sorbic acid stress activates the Candida glabrata high osmolarity glycerol MAP kinase pathway.[Pubmed:24324463]
Front Microbiol. 2013 Nov 26;4:350.
Weak organic acids such as Sorbic acid are important food preservatives and powerful fungistatic agents. These compounds accumulate in the cytosol and disturb the cellular pH and energy homeostasis. Candida glabrata is in many aspects similar to Saccharomyces cerevisiae. However, with regard to confrontation to Sorbic acid, two of the principal response pathways behave differently in C. glabrata. In yeast, Sorbic acid stress causes activation of many genes via the transcription factors Msn2 and Msn4. The C. glabrata homologs CgMsn2 and CgMsn4 are apparently not activated by Sorbic acid. In contrast, in C. glabrata the high osmolarity glycerol (HOG) pathway is activated by Sorbic acid. Here we show that the MAP kinase of the HOG pathway, CgHog1, becomes phosphorylated and has a function for weak acid stress resistance. Transcript profiling of weak acid treated C. glabrata cells suggests a broad and very similar response pattern of cells lacking CgHog1 compared to wild type which is over lapping with but distinct from S. cerevisiae. The PDR12 gene was the highest induced gene in both species and it required CgHog1 for full expression. Our results support flexibility of the response cues for general stress signaling pathways, even between closely related yeasts, and functional extension of a specific response pathway.
Impact of sorbic acid on germination and outgrowth heterogeneity of Bacillus cereus ATCC 14579 spores.[Pubmed:23001664]
Appl Environ Microbiol. 2012 Dec;78(23):8477-80.
Population heterogeneity complicates the predictability of the outgrowth kinetics of individual spores. Flow cytometry sorting and monitoring of the germination and outgrowth of single dormant spores allowed the quantification of acid-induced spore population heterogeneity at pH 5.5 and in the presence of Sorbic acid. This showed that germination efficiency was not a good predictor for heterogeneity in final outgrowth.


