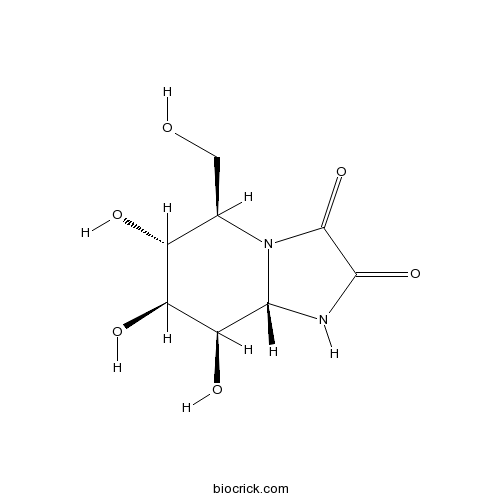
KifunensineCAS# 109944-15-2 |
- Vatalanib (PTK787) 2HCl
Catalog No.:BCC1111
CAS No.:212141-51-0
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
- Lenvatinib (E7080)
Catalog No.:BCC1172
CAS No.:417716-92-8
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- Brivanib (BMS-540215)
Catalog No.:BCC1231
CAS No.:649735-46-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 109944-15-2 | SDF | Download SDF |
| PubChem ID | 130611 | Appearance | Powder |
| Formula | C8H12N2O6 | M.Wt | 232.19 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water with sonication | ||
| Chemical Name | (5R,6R,7S,8R,8aS)-6,7,8-trihydroxy-5-(hydroxymethyl)-1,5,6,7,8,8a-hexahydroimidazo[1,2-a]pyridine-2,3-dione | ||
| SMILES | C(C1C(C(C(C2N1C(=O)C(=O)N2)O)O)O)O | ||
| Standard InChIKey | OIURYJWYVIAOCW-PQMKYFCFSA-N | ||
| Standard InChI | InChI=1S/C8H12N2O6/c11-1-2-3(12)4(13)5(14)6-9-7(15)8(16)10(2)6/h2-6,11-14H,1H2,(H,9,15)/t2-,3-,4+,5+,6+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of class I α-mannosidases that inhibits glycoprotein processing. Inhibits human endoplasmic reticulum α-1,2-mannosidase I and Golgi Class I mannosidases IA, IB and IC with Ki values of 130 and 23 nM respectively. |

Kifunensine Dilution Calculator

Kifunensine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3068 mL | 21.5341 mL | 43.0682 mL | 86.1364 mL | 107.6704 mL |
| 5 mM | 0.8614 mL | 4.3068 mL | 8.6136 mL | 17.2273 mL | 21.5341 mL |
| 10 mM | 0.4307 mL | 2.1534 mL | 4.3068 mL | 8.6136 mL | 10.767 mL |
| 50 mM | 0.0861 mL | 0.4307 mL | 0.8614 mL | 1.7227 mL | 2.1534 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4307 mL | 0.8614 mL | 1.0767 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sodium prasterone sulfate
Catalog No.:BCC9149
CAS No.:1099-87-2
- Granisetron
Catalog No.:BCC1601
CAS No.:109889-09-0
- CGS 9343B
Catalog No.:BCC7303
CAS No.:109826-27-9
- Kaempferol 5,7,4'-trimethyl ether
Catalog No.:BCN6587
CAS No.:1098-92-6
- Harmalidine
Catalog No.:BCN5889
CAS No.:109794-97-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- 8 beta-(4-Acetoxy-5-hydroxytigloyloxy)costunolide
Catalog No.:BCN7123
CAS No.:109770-86-7
- cis-Dehydroosthol
Catalog No.:BCN4735
CAS No.:109741-40-4
- Murraol
Catalog No.:BCN5888
CAS No.:109741-38-0
- MLN 2480
Catalog No.:BCC1771
CAS No.:1096708-71-2
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- Succinic acid
Catalog No.:BCN5890
CAS No.:110-15-6
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
A practical synthesis of kifunensine analogues as inhibitors of endoplasmic reticulum alpha-mannosidase I.[Pubmed:16292820]
J Org Chem. 2005 Nov 25;70(24):9892-904.
[reaction: see text] A practical synthesis of the potent class I alpha-mannosidase inhibitor Kifunensine (1) beginning from the inexpensive and readily available starting material L-ascorbic acid (15) is described. The protected amino-alcohol ((2R,3R,4R,5R)-5-amino-2,3:4,6-diisopropylidenedioxyhexanol, 11) served as a key intermediate from which several N-1 substituted Kifunensine analogues (including N-methyl, N-cyclohexyl, and N-bis(hydroxymethyl)methyl) and 2-desoxaKifunensine analogues (including N-H and N-methyl) were prepared and screened for inhibition of human endoplasmic reticulum alpha-mannosidase I (ER Man I) and mouse Golgi alpha-mannosidase IA (Golgi Man IA). In addition, several pseudodisaccharide Kifunensine analogues in which a mannose residue was tethered to N-1 of Kifunensine via a two-, three-, or four-carbon linker and an affinity-bound Kifunensine analogue were also prepared and evaluated for biological activity. While the synthesized N-1 kifunesine analogues were found to be less potent inhibitors of Class I alpha-mannosidases than kifuensine itself, the bis(hydroxymethyl)methylKifunensine analogue 6 was shown to selectively inhibit ER Man I over Golgi Man IA.
Synthesis of kifunensine thioanalogs and their inhibitory activities against HIV-RT and alpha-mannosidase.[Pubmed:23159373]
Carbohydr Res. 2013 Jan 10;365:1-8.
An efficient and practical synthesis of Kifunensine thioanalogs 1a-c was reported. The bicyclic azasugars fused thiazolidin-4-one 4a-c as key intermediates were first synthesized in good yields of 74-80% via one-pot tandem Staudinger/aza-Wittig/cyclization by using the pivotal azidosugars 3a and 3b derived from D-mannose. Followed by double Pummerer rearrangements and deprotection, the target thioKifunensine 1a and its epimers 1b and 1c were obtained in good yields. Compounds 1a-c were preliminary evaluated for their HIV-RT and alpha-mannosidase (Jack bean) inhibitory activities. The results showed that such compounds exhibited significant anti-HIV-RT inhibitory activity but poor inhibitory against alpha-mannosidase. To gain further insight into the inhibitory mechanism of compounds 1a-c, the analog compounds 9a-c were also prepared after deprotection from 4a-c, respectively. Activity comparison between compounds 1a-c and 9a-c suggests that the better activities of 1a-c than those of the 9a-c is possibly due to the additional carbonyl at thiazolidine-4-one ring in fused bicyclic azasugars.
Use of the alpha-mannosidase I inhibitor kifunensine allows the crystallization of apo CTLA-4 homodimer produced in long-term cultures of Chinese hamster ovary cells.[Pubmed:21795794]
Acta Crystallogr Sect F Struct Biol Cryst Commun. 2011 Jul 1;67(Pt 7):785-9.
Glycoproteins present problems for structural analysis since they often have to be glycosylated in order to fold correctly and because their chemical and conformational heterogeneity generally inhibits crystallization. It is shown that the alpha-mannosidase I inhibitor Kifunensine, which has previously been used for the purpose of glycoprotein crystallization in short-term (3-5 d) cultures, is apparently stable enough to be used to produce highly endoglycosidase H-sensitive glycoprotein in long-term (3-4 week) cultures of stably transfected Chinese hamster ovary (CHO) cells. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry-based analysis of the extracellular region of the cytotoxic T-lymphocyte antigen 4 (CTLA-4; CD152) homodimer expressed in long-term CHO cell cultures in the presence of Kifunensine revealed that the inhibitor restricted CTLA-4 glycan processing to Man9GlcNAc2 and Man5GlcNAc2 structures. Complex-type glycans were undetectable, suggesting that the inhibitor was active for the entire duration of the cultures. Endoglycosidase treatment of the homodimer yielded protein that readily formed orthorhombic crystals with unit-cell parameters a=43.9, b=51.5, c=102.9 A and space group P2(1)2(1)2(1) that diffracted to Bragg spacings of 1.8 A. The results indicate that Kifunensine will be effective in most, if not all, transient and long-term mammalian cell-based expression systems.
A combined STD-NMR/molecular modeling protocol for predicting the binding modes of the glycosidase inhibitors kifunensine and salacinol to Golgi alpha-mannosidase II.[Pubmed:15865418]
Biochemistry. 2005 May 10;44(18):6729-37.
A combined STD-NMR/molecular modeling protocol to probe the binding modes of the glycosidase inhibitors Kifunensine and salacinol to Drosophila melanogaster Golgi alpha-mannosidase II (dGMII) was tested. Saturation-transfer difference (STD) NMR experiments were carried out for the complexes of dGMII with these two inhibitors. The program AutoDock 3.0 was then used to optimize the interactions of the inhibitors with the residues in the active site of dGMII. Theoretical STD effects of the ligand protons in the complexes were calculated for the different binding modes with the recently developed CORCEMA-ST protocol. Comparison of experimental and theoretical effects then permitted selection of the likely binding modes of the ligands. The more rigid Kifunensine was used initially to test the protocol. Excellent correlation between experimental and theoretical data was obtained for one of the binding modes that also corresponded to that observed in the crystal structure of the complex. The protocol was then extended to the more flexible salacinol. For the selected binding mode, good correlation of experimental and theoretical data for the five-membered ring was obtained; however, poor correlation for protons on the acyclic chain was obtained, suggesting flexibility in this portion of the molecule. Comparison of the selected binding mode with that from a crystal structure of salacinol with dGMII showed excellent superimposition of the five-membered ring but another orientation of the acyclic chain. The results suggest that reliable structural binding modes of a ligand to protein in aqueous solution can be provided with the combined use of STD-NMR spectroscopy, molecular modeling, and CORCEMA-ST calculations, although highly flexible portions of the ligand may be poorly defined.


