Tivozanib (AV-951)VEGFR inhibitor,potent and selective CAS# 475108-18-0 |
- c-Met inhibitor 1
Catalog No.:BCC1488
CAS No.:1357072-61-7
- PHA-665752
Catalog No.:BCC1181
CAS No.:477575-56-7
- Cabozantinib (XL184, BMS-907351)
Catalog No.:BCC1264
CAS No.:849217-68-1
- AMG-458
Catalog No.:BCC3721
CAS No.:913376-83-7
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- PF-04217903 methanesulfonate
Catalog No.:BCC1849
CAS No.:956906-93-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475108-18-0 | SDF | Download SDF |
| PubChem ID | 9911830 | Appearance | Powder |
| Formula | C22H19ClN4O5 | M.Wt | 454.86 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AV-951; KRN951 | ||
| Solubility | DMSO : 25 mg/mL (54.96 mM; Need ultrasonic) | ||
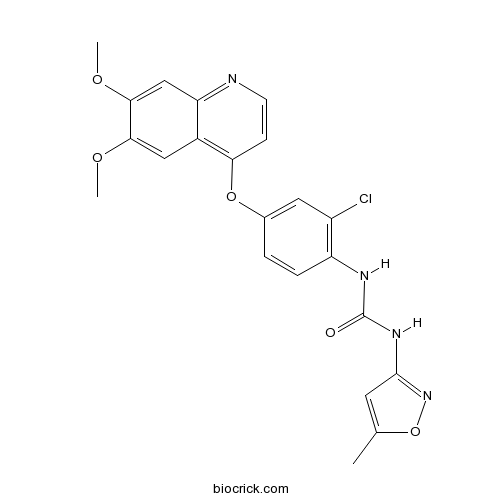
| Chemical Name | 1-[2-chloro-4-(6,7-dimethoxyquinolin-4-yl)oxyphenyl]-3-(5-methyl-1,2-oxazol-3-yl)urea | ||
| SMILES | CC1=CC(=NO1)NC(=O)NC2=C(C=C(C=C2)OC3=C4C=C(C(=CC4=NC=C3)OC)OC)Cl | ||
| Standard InChIKey | SPMVMDHWKHCIDT-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H19ClN4O5/c1-12-8-21(27-32-12)26-22(28)25-16-5-4-13(9-15(16)23)31-18-6-7-24-17-11-20(30-3)19(29-2)10-14(17)18/h4-11H,1-3H3,(H2,25,26,27,28) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Tivozanib is an inhibitor of tyrosine kinase with IC50 value of 160 pM for VEGFR-2. | |||||
| Targets | VEGFR-2 | |||||
| IC50 | 160 pM | |||||

Tivozanib (AV-951) Dilution Calculator

Tivozanib (AV-951) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1985 mL | 10.9924 mL | 21.9848 mL | 43.9696 mL | 54.962 mL |
| 5 mM | 0.4397 mL | 2.1985 mL | 4.397 mL | 8.7939 mL | 10.9924 mL |
| 10 mM | 0.2198 mL | 1.0992 mL | 2.1985 mL | 4.397 mL | 5.4962 mL |
| 50 mM | 0.044 mL | 0.2198 mL | 0.4397 mL | 0.8794 mL | 1.0992 mL |
| 100 mM | 0.022 mL | 0.1099 mL | 0.2198 mL | 0.4397 mL | 0.5496 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Tivozanib is an inhibitor of tyrosine kinase with IC50 value of 160 pmol/L against VEGFR-2 [1].
Tivozanib is a quinoline-urea derivative. As a 2nd generation TKI, it has picomolar potency against VEGFR-1, -2 and -3, and minimal c-kit inhibition. Among this, Tivozanib has demonstrated a VEGFR-2 potency 2 orders of magnitude greater than sunitinib, sorafenib or pazopanib and a lower relative extent of off-target inhibition [1]. Tivozanib also shows to inhibit phosphorylation of the kinases PDGFRß and C-KIT at nanomolar level in cellular assays [2].
Tivozanib has shown antitumor activity in RCC xenograft models in addition to several other solid tumor models leading to its evaluation in clinical testing. The safety and efficacy of Tivozanib has been evaluated in several Phase I and Phase II trials. To compare the front-line use of tivozanib to sorafenib, a pivotal randomized Phase III trial has also been reported [3].
References:
[1] M.N. Fishman, S. Srinivas, R.J. Hauke, R.J. Amato, B. Esteves, M.M. Cotreau, A.L. Strahs, W.J. Slichenmyer, P. Bhargava, F.F. Kabbinavar. Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma. European Journal of Cancer. 2013(49):2841-2850.
[2] Viktor Grunwald, Axel Stuart Merseburger. The progression free survival-plateau with vascular endothelial growth factor receptor inhibitors – Is there more to come? European Journal of Cancer. 2013(49):2504-2511.
[3] C Lance Cowey. Profile of tivozanib and its potential for the treatment of advanced renal cell carcinoma. Drug Design, Development and Therapy. 2013 (7): 519-527.
- NS 304
Catalog No.:BCC7661
CAS No.:475086-01-2
- Nuciferine
Catalog No.:BCN1223
CAS No.:475-83-2
- Glaucine
Catalog No.:BCN2550
CAS No.:475-81-0
- Aristolochic acid B
Catalog No.:BCN6263
CAS No.:475-80-9
- Liriodenine
Catalog No.:BCN5532
CAS No.:475-75-2
- (+)-Isocorynoline
Catalog No.:BCN2361
CAS No.:475-67-2
- H-N-Me-Pro-OH
Catalog No.:BCC3351
CAS No.:475-11-6
- SB 657510
Catalog No.:BCC7713
CAS No.:474960-44-6
- ST 91
Catalog No.:BCC7436
CAS No.:4749-61-5
- 2,16-Kauranediol 2-O-beta-D-allopyranoside
Catalog No.:BCN1436
CAS No.:474893-07-7
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- N-Methylsarpagine methosalt
Catalog No.:BCN5530
CAS No.:47418-70-2
- ZSTK474
Catalog No.:BCC3657
CAS No.:475110-96-4
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- 2-Methylthioadenosine diphosphate trisodium salt
Catalog No.:BCC5794
CAS No.:475193-31-8
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
Phase Ib study of tivozanib (AV-951) in combination with temsirolimus in patients with renal cell carcinoma.[Pubmed:23726267]
Eur J Cancer. 2013 Sep;49(13):2841-50.
BACKGROUND: Tivozanib is a potent and selective tyrosine kinase inhibitor of vascular endothelial growth factor receptors (VEGFR)-1, -2 and -3, with a long half-life. Tivozanib has demonstrated clinical activity and acceptable tolerability in renal cell carcinoma (RCC). This phase Ib study determined the recommended phase II dose (RP2D) and evaluated the safety and clinical activity of tivozanib plus temsirolimus, a mammalian target of rapamycin inhibitor. PATIENTS AND METHODS: Patients with advanced RCC were administered open-label tivozanib 0.5, 1.0 or 1.5mg/d orally (3 weeks on/1 week off) and temsirolimus 15 or 25 mg/week intravenously in a 3+3 dose-escalation design and subsequent expansion cohort. RESULTS: Of 27 patients treated, 20 patients had received >/= 1 prior VEGF-targeted therapy. No dose-limiting toxicities occurred; the RP2D was determined to be tivozanib 1.5mg/d plus temsirolimus 25mg/week. Combination of tivozanib plus temsirolimus demonstrated acceptable tolerability and suggested no synergistic toxicity. The most common grade
Multicenter phase II study of tivozanib (AV-951) and everolimus (RAD001) for patients with refractory, metastatic colorectal cancer.[Pubmed:23580238]
Oncologist. 2013;18(4):377-8.
BACKGROUND: Treatments that target the vascular endothelial growth factor (VEGF) pathway have efficacy in colorectal cancer. We evaluated tolerability and efficacy of tivozanib (an oral VEGF receptor-1, -2, -3 inhibitor) plus everolimus (an oral mammalian target of rapamycin inhibitor). METHODS: The phase Ib study followed a 3 + 3 dose-escalation design with three dose levels. The primary objective in the follow-on phase II study was improvement in 2-month progression-free survival (PFS) from 30% (historical benchmark) to 50% in patients with refractory, metastatic colorectal cancer. RESULTS: Dose-limiting toxicities in the phase Ib study were grade 3 fatigue and dehydration. Oral tivozanib (1 mg daily for 3 of 4 weeks) and oral everolimus (10 mg daily continuously) were advanced to a 40-patient phase II study. The most common grade 3-4 adverse events were thrombocytopenia and hypophosphatemia. The 2-month PFS rate was 50%, with 20 of 40 patients having stable disease (SD). Seven (18%) patients were treated for >/=6 months. Median PFS and overall survival (OS) times were 3.0 months (95% confidence interval [CI]: 1.9-3.6 months) and 5.6 months (95% CI: 4.4-10.6 months), respectively. Patients who developed grade 1+ hypertension had increased SD rates (65.2% vs. 29.4%) and longer OS times (10.6 vs. 3.7 months). CONCLUSIONS: The oral combination of tivozanib and everolimus was well tolerated, with stable disease achieved in 50% of patients with refractory, metastatic colorectal cancer.


