AleglitazarPPARα/PPARγagonist CAS# 475479-34-6 |
- GW1929
Catalog No.:BCC1611
CAS No.:196808-24-9
- Balaglitazone
Catalog No.:BCC1395
CAS No.:199113-98-9
- Inolitazone
Catalog No.:BCC1652
CAS No.:223132-37-4
- Inolitazone dihydrochloride
Catalog No.:BCC1653
CAS No.:223132-38-5
- L-165041
Catalog No.:BCC1687
CAS No.:79558-09-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475479-34-6 | SDF | Download SDF |
| PubChem ID | 10274777 | Appearance | Powder |
| Formula | C24H23NO5S | M.Wt | 437.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R1439; RO0728804 | ||
| Solubility | DMSO : ≥ 50 mg/mL (114.28 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
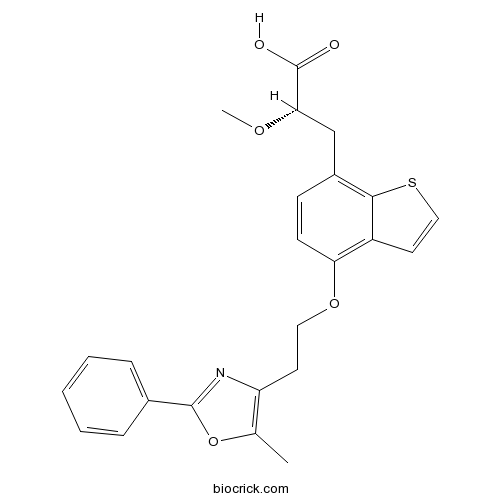
| Chemical Name | (2S)-2-methoxy-3-[4-[2-(5-methyl-2-phenyl-1,3-oxazol-4-yl)ethoxy]-1-benzothiophen-7-yl]propanoic acid | ||
| SMILES | CC1=C(N=C(O1)C2=CC=CC=C2)CCOC3=C4C=CSC4=C(C=C3)CC(C(=O)O)OC | ||
| Standard InChIKey | DAYKLWSKQJBGCS-NRFANRHFSA-N | ||
| Standard InChI | InChI=1S/C24H23NO5S/c1-15-19(25-23(30-15)16-6-4-3-5-7-16)10-12-29-20-9-8-17(14-21(28-2)24(26)27)22-18(20)11-13-31-22/h3-9,11,13,21H,10,12,14H2,1-2H3,(H,26,27)/t21-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aleglitazar (R1439) is a dual agonist of peroxisome proliferator-activated receptor (PPAR), with affinity to PPARα and PPARγ. | |||||
| Targets | PPAR | |||||

Aleglitazar Dilution Calculator

Aleglitazar Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2857 mL | 11.4283 mL | 22.8566 mL | 45.7132 mL | 57.1416 mL |
| 5 mM | 0.4571 mL | 2.2857 mL | 4.5713 mL | 9.1426 mL | 11.4283 mL |
| 10 mM | 0.2286 mL | 1.1428 mL | 2.2857 mL | 4.5713 mL | 5.7142 mL |
| 50 mM | 0.0457 mL | 0.2286 mL | 0.4571 mL | 0.9143 mL | 1.1428 mL |
| 100 mM | 0.0229 mL | 0.1143 mL | 0.2286 mL | 0.4571 mL | 0.5714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Aleglitazar is a potent dual agonist of peroxisome proliferator-activated receptor (PPAR) α/γ with IC50 values of 38 and 19 nM, respectively [1].
PPAR are nuclear hormone receptors. They work as ligand-activated transcription factors and regulate gene expression with co-activators and co-repressors. PPARα usually takes participate in energy homeostasis while PPARγ plays critical roles in glucose homeostasis and insulin sensitivity. So far the agonists of PPAR α/γ are used in the treatment for dyslipidemia or typeII diabetes. Aleglitazar is a balanced and potent co-agonist of both PPARα and PPARγ based on α-alkoxyacid therefore is thought to be a probable opportunity to treat hyperglycemia and diabetes [1].
Aleglitazar showed similar affinities for both PPARα and PPARγ with IC50 values of 38 and 19 nM respectively in the radioligand binding assays. In the transcriptional assays using luciferase transcriptional reporter gene, aleglitazar showed EC50 values of 50 and 21 nM for PPARα and PPARγ, respectively. Aleglitazar had lower maximum activity towards PPARαthan other agonists suggesting that it was a partial PPARαagonist [1 and 2].
In the primate model using rhesus monkeys, the oral administration of aleglitazar at dose of 0.03 mg/kg per day for 42 days resulted in significantly increased glucose disposal rate (7.8 to 12.5 mg/kg fat-free mass) and decreased TG levels (328 to 36 mg/dL). The FPG levels were reduced by aleglitazarfrom 89 to 75 mg/dL. Aleglitazar increased the levels of HDL-C by 125% and reduced LDL-C by 41%. Besides that, aleglitazar treatment increased the levels of ApoA-I and ApoA-II. In the euglycemic clamp study using Zuckerfa/farats, aleglitazar behaved more effectively than rosiglitazone and farglitazar [1 and 3].
References:
[1] Bénardeau A, Benz J, Binggeli A, Blum D, Boehringer M, Grether U, Hilpert H, Kuhn B, Märki HP, Meyer M, Püntener K, Raab S, Ruf A, Schlatter D, Mohr P. Aleglitazar, a new, potent, and balanced dual PPARalpha/gamma agonist for the treatment of type II diabetes. Bioorg Med Chem Lett. 2009 May 1;19(9):2468-73. doi: 10.1016/j.bmcl.2009.03.036..
[2] Dietz M, Mohr P, Kuhn B, Maerki HP, Hartman P, Ruf A, Benz J, Grether U, Wright MB. Comparative molecular profiling of the PPARα/γ activator aleglitazar: PPAR selectivity, activity and interaction with cofactors. ChemMedChem. 2012 Jun;7(6):1101-11.
[3] Hansen BC, Tigno XT, Bénardeau A, Meyer M, Sebokova E, Mizrahi J. Effects of aleglitazar, a balanced dual peroxisome proliferator-activated receptor α/γ agonist on glycemic and lipid parameters in a primate model of the metabolic syndrome. Cardiovasc Diabetol. 2011 Jan 20;10:7.
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- 2-Methylthioadenosine diphosphate trisodium salt
Catalog No.:BCC5794
CAS No.:475193-31-8
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- ZSTK474
Catalog No.:BCC3657
CAS No.:475110-96-4
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- NS 304
Catalog No.:BCC7661
CAS No.:475086-01-2
- Nuciferine
Catalog No.:BCN1223
CAS No.:475-83-2
- Glaucine
Catalog No.:BCN2550
CAS No.:475-81-0
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Xylotriose
Catalog No.:BCN8428
CAS No.:47592-59-6
- Lycorine
Catalog No.:BCN2409
CAS No.:476-28-8
- Chelidonine
Catalog No.:BCN2463
CAS No.:476-32-4
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
- Corydine
Catalog No.:BCN2669
CAS No.:476-69-7
- Boldine
Catalog No.:BCN5534
CAS No.:476-70-0
- VO-Ohpic trihydrate
Catalog No.:BCC2043
CAS No.:476310-60-8
- Eupaglehnin C
Catalog No.:BCN7118
CAS No.:476630-49-6
Aleglitazar, a dual peroxisome proliferator-activated receptor-alpha and -gamma agonist, protects cardiomyocytes against the adverse effects of hyperglycaemia.[Pubmed:28111985]
Diab Vasc Dis Res. 2017 Mar;14(2):152-162.
PURPOSE: To assess the effects of Aleglitazar on hyperglycaemia-induced apoptosis. METHODS: We incubated human cardiomyocytes, cardiomyocytes from cardiac-specific peroxisome proliferator-activated receptor-gamma knockout or wild-type mice in normoglycaemic or hyperglycaemic conditions (glucose 25 mM). Cells were treated with different concentrations of Aleglitazar for 48 h. We measured viability, apoptosis, caspase-3 activity, cytochrome-C release, total antioxidant capacity and reactive oxygen species formation in the treated cardiomyocytes. Human cardiomyocytes were transfected with short interfering RNA against peroxisome proliferator-activated receptor-alpha or peroxisome proliferator-activated receptor-gamma. RESULTS: Aleglitazar attenuated hyperglycaemia-induced apoptosis, caspase-3 activity and cytochrome-C release and increased viability in human cardiomyocyte, cardiomyocytes from cardiac-specific peroxisome proliferator-activated receptor-gamma knockout and wild-type mice. Hyperglycaemia reduced the antioxidant capacity and Aleglitazar significantly blunted this effect. Hyperglycaemia-induced reactive oxygen species production was attenuated by Aleglitazar in both human cardiomyocyte and wild-type mice cardiomyocytes. Aleglitazar improved cell viability in cells exposed to hyperglycaemia. The protective effect was partially blocked by short interfering RNA against peroxisome proliferator-activated receptor-alpha alone and short interfering RNA against peroxisome proliferator-activated receptor-gamma alone and completely blocked by short interfering RNA to both peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma. CONCLUSION: Aleglitazar protects cardiomyocytes against hyperglycaemia-induced apoptosis by combined activation of both peroxisome proliferator-activated receptor-alpha and peroxisome proliferator-activated receptor-gamma in a short-term vitro model.
Aleglitazar, a Balanced Dual PPARalpha and -gamma Agonist, Protects the Heart Against Ischemia-Reperfusion Injury.[Pubmed:26861490]
Cardiovasc Drugs Ther. 2016 Apr;30(2):129-41.
PURPOSE: To evaluate whether Aleglitazar (Ale), a dual PPARalpha/gamma agonist, has additive effects on myocardial protection against ischemia-reperfusion injury. METHODS: Human cardiomyocytes (HCMs), cardiomyocytes from cardiac-specific PPARgamma knockout (MCM-PPARgamma (CKO) ) or wild type (MCM-WT) mice were incubated with different concentrations of Ale, and subjected to simulated ischemia-reperfusion (SIR) or normoxic conditions (NSIR). Cell viability, apoptosis and caspase-3 activity were determined. HCMs were transfected with siRNA against PPARalpha (siPPARalpha) or PPARgamma (siPPARgamma) followed by incubation with Ale. PPARalpha/gamma DNA binding capacity was measured. Cell viability, apoptosis and levels of P-AKT and P-eNOS were assessed. Infarct size following 30 min coronary artery occlusion and 24 h reperfusion were assessed in WT and db/db diabetic mice following 3-day pretreatment with vehicle, Ale or glimeperide. RESULTS: Ale (at concentrations of 150-600 nM) increased cell viability and reduced apoptosis in HCMs, MCM-WT and MCM-PPAR (CKO) exposed to SIR. In HCM, the protective effect was partially blocked by siPPARalpha alone or siPPARgamma alone, and completely blocked by siPPARalpha+siPPARgamma. Ale increased P-Akt/P-eNOS in HCMs. P-Akt or P-eNOS levels were decreased when PPARalpha alone, PPARgamma alone and especially when both were knocked down. Peritoneal GTTs revealed that db/db mice had developed impaired glucose tolerance and insulin sensitivity, which were normalized by Ale or glimepiride treatment. Ale, but not glimepiride, limited infarct size in both WT and diabetic mice after ischemia-reperfusion. CONCLUSIONS: Ale protects against myocardial apoptosis caused by hypoxia-reoxygenation in vitro and reduces infarct size in vivo.
Therapeutic potential of the dual peroxisome proliferator activated receptor (PPAR)alpha/gamma agonist aleglitazar in attenuating TNF-alpha-mediated inflammation and insulin resistance in human adipocytes.[Pubmed:26976796]
Pharmacol Res. 2016 May;107:125-136.
Adipose tissue inflammation is a mechanistic link between obesity and its related sequelae, including insulin resistance and type 2 diabetes. Dual ligands of peroxisome proliferator activated receptor (PPAR)alpha and gamma, combining in a single molecule the metabolic and inflammatory-regulatory properties of alpha and gamma agonists, have been proposed as a promising therapeutic strategy to antagonize adipose tissue inflammation. Here we investigated the effects of the dual PPARalpha/gamma agonist Aleglitazar on human adipocytes challenged with inflammatory stimuli. Human Simpson-Golabi-Behmel syndrome (SGBS) adipocytes were treated with Aleglitazar or - for comparison - the selective agonists for PPARalpha or gamma fenofibrate or rosiglitazone, respectively, for 24h before stimulation with TNF-alpha. Aleglitazar, at concentrations as low as 10nmol/L, providing the half-maximal transcriptional activation of both PPARalpha and PPARgamma, reduced the stimulated expression of several pro-inflammatory mediators including interleukin (IL)-6, the chemokine CXC-L10, and monocyte chemoattractant protein (MCP)-1. Correspondingly, media from adipocytes treated with Aleglitazar reduced monocyte migration, consistent with suppression of MCP-1 secretion. Under the same conditions, Aleglitazar also reversed the TNF-alpha-mediated suppression of insulin-stimulated ser473 Akt phosphorylation and decreased the TNF-alpha-induced ser312 IRS1 phosphorylation, two major switches in insulin-mediated metabolic activities, restoring glucose uptake in insulin-resistant adipocytes. Such effects were similar to those obtainable with a combination of single PPARalpha and gamma agonists. In conclusion, Aleglitazar reduces inflammatory activation and dysfunction in insulin signaling in activated adipocytes, properties that may benefit diabetic and obese patients. The effect of Aleglitazar was consistent with dual PPARalpha and gamma agonism, but with no evidence of synergism.
Non-clinical safety evaluation and risk assessment to human of aleglitazar, a dual PPAR alpha/gamma agonist, and its major human metabolite.[Pubmed:28274810]
Regul Toxicol Pharmacol. 2017 Jun;86:107-116.
The non-clinical safety profile of Aleglitazar, a peroxisome proliferator activated receptor alpha/gamma agonist, and its major human metabolite M6 was studied in a complete package consisting of drug metabolism and pharmacokinetics characterization, safety pharmacology, genotoxicity, repeat dose toxicity, reproductive toxicity and carcinogenicity studies. These studies identified the following main targets similar to other PPAR agonists: red blood cell parameters, liver, heart, kidney, ovaries, testes, bone marrow, adipose tissue, and fluid accumulation. Additionally, and in the 12-month monkey study only, an increased incidence of generalized hair loss/thinning was observed in all groups including controls. In the rat carcinogenicity study there was no statistically significant increase in tumors. In the mouse carcinogenicity study, there was an increased incidence of angiomatous tumors and there were three males with gallbladder adenoma. No relevant compound-related effects were observed in safety pharmacology, genotoxicity, and a 28-day immunotoxicity rat study. Effects observed in reproductive toxicity studies were similar to those known for other PPARgamma agonists. Separate studies with the human metabolite M6 did not reveal findings that would prevent human dosing. Overall, the results from the non-clinical safety studies conducted with Aleglitazar and the human metabolite M6 were considered to support the clinical Phase 3 program.


