BoldineCAS# 476-70-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 476-70-0 | SDF | Download SDF |
| PubChem ID | 10154 | Appearance | Grey-beige powder |
| Formula | C19H21NO4 | M.Wt | 327.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | Soluble in ethanol; very slightly soluble in water | ||
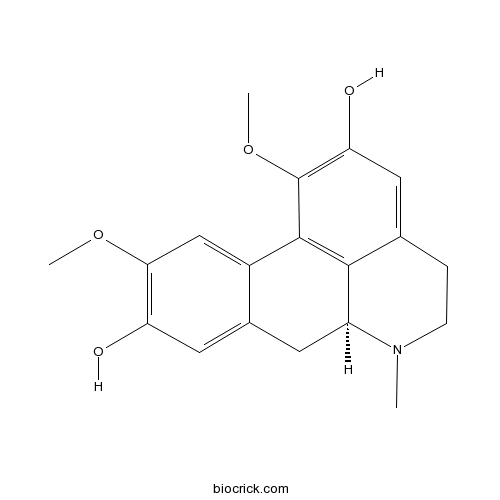
| Chemical Name | (6aS)-1,10-dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-2,9-diol | ||
| SMILES | CN1CCC2=CC(=C(C3=C2C1CC4=CC(=C(C=C43)OC)O)OC)O | ||
| Standard InChIKey | LZJRNLRASBVRRX-ZDUSSCGKSA-N | ||
| Standard InChI | InChI=1S/C19H21NO4/c1-20-5-4-10-7-15(22)19(24-3)18-12-9-16(23-2)14(21)8-11(12)6-13(20)17(10)18/h7-9,13,21-22H,4-6H2,1-3H3/t13-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Boldine displays anti-cancer, cytoprotective , anti-oxidant and anti-inflammatory activities. Boldine reduces oxidative stress and improves endothelium-dependent relaxation in aortas of diabetic mice largely through inhibiting ROS overproduction associated with Ang II-mediated BMP4-dependent mechanisms. Boldine may attenuate the catecholamine oxidation-induced brain mitochondrial dysfunction and decrease the dopamine-induced death of PC12 cells through a scavenging action on reactive oxygen species and inhibition of melanin formation and thiol oxidation. |
| Targets | FXR | ROS | GSK-3 | Akt | ERK | NOS | Calcium Channel |
| In vitro | Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3β.[Pubmed: 24239461 ]Urol Oncol. 2014 Jan;32(1):36.e1-9.The objective of the present study was to evaluate the effect and underlying mechanisms of Boldine, an aporphine alkaloid of Peumus boldus, on bladder cancer proliferation and cell death. METHODS: Sulforhodamine B assay, Tetrazolium reduction assay, Flow Cytometry Analysis, Ecto-5'-nucleotidase activity and Western blot assay were performed. RESULTS: The results showed that Boldine was able to reduce cell viability and cell proliferation in T24 cells. In addition, Boldine arrests the cell cycle at G2/M-phase and cause cell death by apoptosis. Boldine-induced inhibition of cell growth and cell cycle arrest appears to be linked to inactivation of extracellular signal-regulated kinase protein (ERK). Additionally, the efficacy of Boldine in apoptosis-induced in T24 cells is correlated with modulation of AKT (inactivation) and glycogen synthase kinase-3β (GSK-3β) (activation) proteins. CONCLUSIONS: The present findings may, in part, explain the therapeutic effects of Boldine for treatment of urinary bladder cancer. Antioxidant and pro-oxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro.[Pubmed: 18590764 ]Neurotoxicology. 2008 Nov;29(6):1136-40.Boldine is one of the most potent natural antioxidants and displays some important pharmacological activities, such as cytoprotective and anti-inflammatory activities, which may arise from its free radical scavenging properties. |
| In vivo | Boldine enhances bile production in rats via osmotic and farnesoid X receptor dependent mechanisms.[Pubmed: 25771127]Toxicol Appl Pharmacol. 2015 May 15;285(1):12-22.Boldine, the major alkaloid from the Chilean Boldo tree, is used in traditional medicine to support bile production, but evidence to support this function is controversial. We analyzed the choleretic potential of Boldine, including its molecular background. Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade.[Pubmed: 23992296]Br J Pharmacol. 2013 Nov;170(6):1190-8.Boldine is a potent natural antioxidant present in the leaves and bark of the Chilean boldo tree. Here we assessed the protective effects of Boldine on endothelium in a range of models of diabetes, ex vivo and in vitro.
|
| Cell Research | Protective effect of boldine on dopamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells.[Pubmed: 11853700]Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations.[Pubmed: 25746354]Chem Biol Interact. 2015 Apr 25;231:27-34.In a preliminary screening study of natural alkaloids, Boldine, an aporphine alkaloid, showed an interesting dose and time dependent anti-proliferative effect in several cancer cell lines. Biochem Pharmacol. 2002 Feb 1;63(3):495-505.Boldine ([S]-2,9-dihydroxy-1,10-dimethoxyaporphine) has been shown to exert antioxidant and anti-inflammatory effects. The present study elucidated the protective effect of Boldine on catecholamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells. |

Boldine Dilution Calculator

Boldine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0544 mL | 15.2718 mL | 30.5437 mL | 61.0874 mL | 76.3592 mL |
| 5 mM | 0.6109 mL | 3.0544 mL | 6.1087 mL | 12.2175 mL | 15.2718 mL |
| 10 mM | 0.3054 mL | 1.5272 mL | 3.0544 mL | 6.1087 mL | 7.6359 mL |
| 50 mM | 0.0611 mL | 0.3054 mL | 0.6109 mL | 1.2217 mL | 1.5272 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3054 mL | 0.6109 mL | 0.7636 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Corydine
Catalog No.:BCN2669
CAS No.:476-69-7
- Ellagic acid
Catalog No.:BCN5533
CAS No.:476-66-4
- Chelidonine
Catalog No.:BCN2463
CAS No.:476-32-4
- Lycorine
Catalog No.:BCN2409
CAS No.:476-28-8
- Xylotriose
Catalog No.:BCN8428
CAS No.:47592-59-6
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- VO-Ohpic trihydrate
Catalog No.:BCC2043
CAS No.:476310-60-8
- Eupaglehnin C
Catalog No.:BCN7118
CAS No.:476630-49-6
- Lushanrubescensin H
Catalog No.:BCN3235
CAS No.:476640-22-9
- 6,9,10-Trihydroxy-7-megastigmen-3-one
Catalog No.:BCN1435
CAS No.:476682-97-0
- 3-(2-Benzothiazolylthio)propionic acid
Catalog No.:BCC8586
CAS No.:4767-00-4
- Boc-Tyr(2-Br-Z)-OH
Catalog No.:BCC3460
CAS No.:47689-67-8
- Lycorenine
Catalog No.:BCN2507
CAS No.:477-19-0
- Demecolcine
Catalog No.:BCC9223
CAS No.:477-30-5
- Samidin
Catalog No.:BCN6665
CAS No.:477-33-8
- Dehydrocostus lactone
Catalog No.:BCN5536
CAS No.:477-43-0
- Beta-Apopicropodophyllin
Catalog No.:BCC1388
CAS No.:477-52-1
- Podophyllotoxinone
Catalog No.:BCN8063
CAS No.:477-49-6
Antioxidant and pro-oxidant properties of boldine on hippocampal slices exposed to oxygen-glucose deprivation in vitro.[Pubmed:18590764]
Neurotoxicology. 2008 Nov;29(6):1136-40.
Boldine is one of the most potent natural antioxidants and displays some important pharmacological activities, such as cytoprotective and anti-inflammatory activities, which may arise from its free radical scavenging properties. Given that the pathogenesis of brain ischemia/reperfusion has been associated with an excessive generation of oxygen free radicals, the aim of this study was to evaluate the neuroproperties of Boldine using hippocampal slices from Wistar rats exposed to oxygen and glucose deprivation (OGD), followed by reoxygenation, to mimic an ischemic condition. The OGD ischemic condition significantly impaired cellular viability, increased lactate dehydrogenase (LDH) leakage and increased free radical generation. In non-OGD slices, incubation with 100microM Boldine significantly increased LDH released into incubation media and decreased mitochondrial activity, suggesting an increase of tissue damage caused by Boldine. However, slices incubated with 10microM Boldine during and after OGD exposure had significantly increased cellular viability with no effect on cell damage. Total reactive antioxidant potential (TRAP) levels measured for this alkaloid showed an antioxidant potential three times higher than Trolox, which acts as a peroxyl radical scavenger. Moreover, Boldine prevented the increase in lipoperoxidation levels induced by ischemia, but higher concentrations potentiated this parameter. These results confirm the potent antioxidant properties of this alkaloid, and add evidence to support the need for further investigations in order to confirm the potential pro-oxidant effects of Boldine at higher doses.
Boldine induces cell cycle arrest and apoptosis in T24 human bladder cancer cell line via regulation of ERK, AKT, and GSK-3beta.[Pubmed:24239461]
Urol Oncol. 2014 Jan;32(1):36.e1-9.
OBJECTIVE: Bladder cancer is one of the most prevalent genitourinary malignancies. Despite active chemotherapy regimens, patients with bladder cancer suffer from a high rate of tumor recurrence. Thus, new approaches and agents to improve quality of life and survival still need to be developed. The objective of the present study was to evaluate the effect and underlying mechanisms of Boldine, an aporphine alkaloid of Peumus boldus, on bladder cancer proliferation and cell death. METHODS: Sulforhodamine B assay, Tetrazolium reduction assay, Flow Cytometry Analysis, Ecto-5'-nucleotidase activity and Western blot assay were performed. RESULTS: The results showed that Boldine was able to reduce cell viability and cell proliferation in T24 cells. In addition, Boldine arrests the cell cycle at G2/M-phase and cause cell death by apoptosis. Boldine-induced inhibition of cell growth and cell cycle arrest appears to be linked to inactivation of extracellular signal-regulated kinase protein (ERK). Additionally, the efficacy of Boldine in apoptosis-induced in T24 cells is correlated with modulation of AKT (inactivation) and glycogen synthase kinase-3beta (GSK-3beta) (activation) proteins. CONCLUSIONS: The present findings may, in part, explain the therapeutic effects of Boldine for treatment of urinary bladder cancer.
Protective effect of boldine on dopamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells.[Pubmed:11853700]
Biochem Pharmacol. 2002 Feb 1;63(3):495-505.
Boldine ([S]-2,9-dihydroxy-1,10-dimethoxyaporphine) has been shown to exert antioxidant and anti-inflammatory effects. The present study elucidated the protective effect of Boldine on catecholamine-induced membrane permeability transition in brain mitochondria and viability loss in PC12 cells. Dopamine (200 microM) and 6-hydroxydopamine (6-OHDA, 100 microM) attenuated Ca(2+) and succinate-induced mitochondrial swelling and membrane potential formation. Boldine (10-100 microM) and 10 microg/mL of superoxide dismutase (SOD) or catalase reduced the effect of catecholamine oxidation on brain mitochondria. Boldine, SOD, and catalase decreased catecholamine-induced mitochondrial cytochrome c release. Antioxidant enzymes attenuated the depressant effect of catecholamines on mitochondrial electron flow, whereas Boldine did not reduce it. Boldine inhibited the catecholamine-induced decrease in thioredoxin reductase activity and the increase in thiol oxidation in mitochondria. It also showed a scavenging action on hydrogen peroxide and hydroxyl radicals and decreased the formation of melanin from dopamine. Boldine and antioxidant enzymes decreased the dopamine-induced cell death, including apoptosis, in PC12 cells. The results suggest that Boldine may attenuate the catecholamine oxidation-induced brain mitochondrial dysfunction and decrease the dopamine-induced death of PC12 cells through a scavenging action on reactive oxygen species and inhibition of melanin formation and thiol oxidation.
Boldine, a natural aporphine alkaloid, inhibits telomerase at non-toxic concentrations.[Pubmed:25746354]
Chem Biol Interact. 2015 Apr 25;231:27-34.
In a preliminary screening study of natural alkaloids, Boldine, an aporphine alkaloid, showed an interesting dose and time dependent anti-proliferative effect in several cancer cell lines. Cytotoxicity of Boldine in human fibroblasts was considerably lower than the telomerase positive embryonic kidney HEK293 and breast cancer MCF-7 and MDA-MB-231 cells. Whether Boldine can inhibit telomerase was investigated here using a modified quantitative real-time telomere repeat amplification protocol (q-TRAP). This test showed that Boldine inhibits telomerase in cells treated with sub-cytotoxic concentrations. Telomerase inhibition occurs via down-regulation of hTERT, the catalytic subunit of the enzyme. Boldine changed the splicing variants of hTERT towards shorter non-functional transcripts as well. A direct interaction of Boldine with the enzyme may also be involved, though thermal FRET method did not detect any substantial interaction between Boldine and synthetic telomere sequences. This study advocates Boldine as a valuable candidate for telomerase-targeted cancer care. This study suggests that derivatives of Boldine could be potent anti-cancer drugs.
Boldine enhances bile production in rats via osmotic and farnesoid X receptor dependent mechanisms.[Pubmed:25771127]
Toxicol Appl Pharmacol. 2015 May 15;285(1):12-22.
Boldine, the major alkaloid from the Chilean Boldo tree, is used in traditional medicine to support bile production, but evidence to support this function is controversial. We analyzed the choleretic potential of Boldine, including its molecular background. The acute- and long-term effects of Boldine were evaluated in rats either during intravenous infusion or after 28-day oral treatment. Infusion of Boldine instantly increased the bile flow 1.4-fold in healthy rats as well as in animals with Mrp2 deficiency or ethinylestradiol induced cholestasis. This effect was not associated with a corresponding increase in bile acid or glutathione biliary excretion, indicating that the effect is not related to stimulation of either bile acid dependent or independent mechanisms of bile formation and points to the osmotic activity of Boldine itself. We subsequently analyzed bile production under conditions of changing biliary excretion of Boldine after bolus intravenous administration and found strong correlations between both parameters. HPLC analysis showed that bile concentrations of Boldine above 10 muM were required for induction of choleresis. Importantly, long-term pretreatment, when the bile collection study was performed 24-h after the last administration of Boldine, also accelerated bile formation despite undetectable levels of the compound in bile. The effect paralleled upregulation of the Bsep transporter and increased biliary clearance of its substrates, bile acids. We consequently confirmed the ability of Boldine to stimulate the Bsep transcriptional regulator, FXR receptor. In conclusion, our study clarified the mechanisms and circumstances surrounding the choleretic activity of Boldine.
Boldine improves endothelial function in diabetic db/db mice through inhibition of angiotensin II-mediated BMP4-oxidative stress cascade.[Pubmed:23992296]
Br J Pharmacol. 2013 Nov;170(6):1190-8.
BACKGROUND AND PURPOSE: Boldine is a potent natural antioxidant present in the leaves and bark of the Chilean boldo tree. Here we assessed the protective effects of Boldine on endothelium in a range of models of diabetes, ex vivo and in vitro. EXPERIMENTAL APPROACH: Vascular reactivity was studied in mouse aortas from db/db diabetic and normal mice. Reactive oxygen species (ROS) production, angiotensin AT1 receptor localization and protein expression of oxidative stress markers in the vascular wall were evaluated by dihydroethidium fluorescence, lucigenin enhanced-chemiluminescence, immunohistochemistry and Western blot respectively. Primary cultures of mouse aortic endothelial cells, exposed to high concentrations of glucose (30 mmol L(-1) ) were also used. KEY RESULTS: Oral treatment (20 mg kg(-1) day(-1) , 7 days) or incubation in vitro with Boldine (1 mumol L(-1) , 12 h) enhanced endothelium-dependent aortic relaxations of db/db mice. Boldine reversed impaired relaxations induced by high glucose or angiotensin II (Ang II) in non-diabetic mouse aortas while it reduced the overproduction of ROS and increased phosphorylation of eNOS in db/db mouse aortas. Elevated expression of oxidative stress markers (bone morphogenic protein 4 (BMP4), nitrotyrosine and AT1 receptors) were reduced in Boldine-treated db/db mouse aortas. Ang II-stimulated BMP4 expression was inhibited by Boldine, tempol, noggin or losartan. Boldine inhibited high glucose-stimulated ROS production and restored the decreased phosphorylation of eNOS in mouse aortic endothelial cells in culture. CONCLUSIONS AND IMPLICATIONS: Boldine reduced oxidative stress and improved endothelium-dependent relaxation in aortas of diabetic mice largely through inhibiting ROS overproduction associated with Ang II-mediated BMP4-dependent mechanisms.


