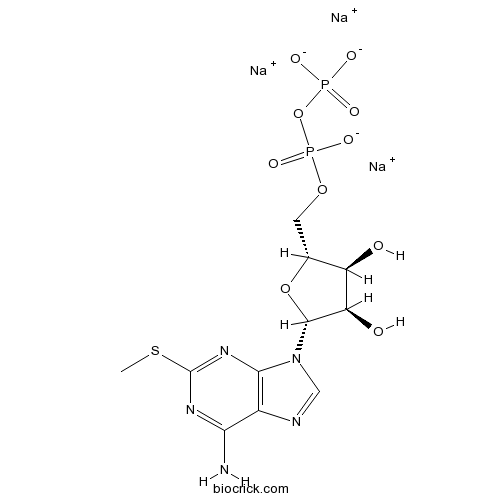
2-Methylthioadenosine diphosphate trisodium saltPotent agonist for P2Y1, P2Y12 and P2Y13 CAS# 475193-31-8 |
- ABT-751 (E7010)
Catalog No.:BCC1085
CAS No.:141430-65-1
- CHM 1
Catalog No.:BCC2387
CAS No.:154554-41-3
- Podophyllotoxin
Catalog No.:BCN5957
CAS No.:518-28-5
- D-64131
Catalog No.:BCC1510
CAS No.:74588-78-6
- CYT997 (Lexibulin)
Catalog No.:BCC4601
CAS No.:917111-44-5
- MPC 6827 hydrochloride
Catalog No.:BCC8040
CAS No.:917369-31-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475193-31-8 | SDF | Download SDF |
| PubChem ID | 52942441 | Appearance | Powder |
| Formula | C11H14N5Na3O10P2S | M.Wt | 539.24 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | 2-(Methylthio)adenosine 5'-diphosphate, 2-Methylthio-ADP, MeSADP | ||
| Solubility | Soluble in water (supplied pre-dissolved at a concentration of 10mM) | ||
| Chemical Name | trisodium;[[(2R,3S,4R,5R)-5-(6-amino-2-methylsulfanylpurin-9-yl)-3,4-dihydroxyoxolan-2-yl]methoxy-oxidophosphoryl] phosphate | ||
| SMILES | CSC1=NC2=C(C(=N1)N)N=CN2C3C(C(C(O3)COP(=O)([O-])OP(=O)([O-])[O-])O)O.[Na+].[Na+].[Na+] | ||
| Standard InChIKey | DYNGCIHMNWOBSU-MSQVLRTGSA-K | ||
| Standard InChI | InChI=1S/C11H17N5O10P2S.3Na/c1-29-11-14-8(12)5-9(15-11)16(3-13-5)10-7(18)6(17)4(25-10)2-24-28(22,23)26-27(19,20)21;;;/h3-4,6-7,10,17-18H,2H2,1H3,(H,22,23)(H2,12,14,15)(H2,19,20,21);;;/q;3*+1/p-3/t4-,6-,7-,10-;;;/m1.../s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent purinergic agonist displaying selectivity for P2Y1, P2Y12 and P2Y13 receptors (pEC50 = 8.29 and 9.05 for P2Y1 and P2Y12, EC50 = 19 nM for P2Y13). Induces aggregation of, and inhibits cAMP accumulation in, platelets in vitro. |

2-Methylthioadenosine diphosphate trisodium salt Dilution Calculator

2-Methylthioadenosine diphosphate trisodium salt Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8545 mL | 9.2723 mL | 18.5446 mL | 37.0892 mL | 46.3615 mL |
| 5 mM | 0.3709 mL | 1.8545 mL | 3.7089 mL | 7.4178 mL | 9.2723 mL |
| 10 mM | 0.1854 mL | 0.9272 mL | 1.8545 mL | 3.7089 mL | 4.6362 mL |
| 50 mM | 0.0371 mL | 0.1854 mL | 0.3709 mL | 0.7418 mL | 0.9272 mL |
| 100 mM | 0.0185 mL | 0.0927 mL | 0.1854 mL | 0.3709 mL | 0.4636 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- ZSTK474
Catalog No.:BCC3657
CAS No.:475110-96-4
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- NS 304
Catalog No.:BCC7661
CAS No.:475086-01-2
- Nuciferine
Catalog No.:BCN1223
CAS No.:475-83-2
- Glaucine
Catalog No.:BCN2550
CAS No.:475-81-0
- Aristolochic acid B
Catalog No.:BCN6263
CAS No.:475-80-9
- Liriodenine
Catalog No.:BCN5532
CAS No.:475-75-2
- (+)-Isocorynoline
Catalog No.:BCN2361
CAS No.:475-67-2
- H-N-Me-Pro-OH
Catalog No.:BCC3351
CAS No.:475-11-6
- SB 657510
Catalog No.:BCC7713
CAS No.:474960-44-6
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
- Aleglitazar
Catalog No.:BCC1337
CAS No.:475479-34-6
- NVP-ADW742
Catalog No.:BCC4553
CAS No.:475488-23-4
- NVP-AEW541
Catalog No.:BCC1180
CAS No.:475489-16-8
- MCL 0020
Catalog No.:BCC6025
CAS No.:475498-26-1
- Isotretinoin
Catalog No.:BCC2284
CAS No.:4759-48-2
- Xylotriose
Catalog No.:BCN8428
CAS No.:47592-59-6
- Lycorine
Catalog No.:BCN2409
CAS No.:476-28-8
- Chelidonine
Catalog No.:BCN2463
CAS No.:476-32-4
High pH-Sensitive Store-Operated Ca(2+) Entry Mediated by Ca(2+) Release-Activated Ca(2+) Channels in Rat Odontoblasts.[Pubmed:29765331]
Front Physiol. 2018 May 1;9:443.
Odontoblasts play a crucial role in dentin formation and sensory transduction following the application of stimuli to the dentin surface. Various exogenous and endogenous stimuli elicit an increase in the intracellular free calcium concentration ([Ca(2+)]i) in odontoblasts, which is mediated by Ca(2+) release from intracellular Ca(2+) stores and/or Ca(2+) influx from the extracellular medium. In a previous study, we demonstrated that the depletion of Ca(2+) stores in odontoblasts activated store-operated Ca(2+) entry (SOCE), a Ca(2+) influx pathway. However, the precise biophysical and pharmacological properties of SOCE in odontoblasts have remained unclear. In the present study, we examined the functional expression and pharmacological properties of Ca(2+) release-activated Ca(2+) (CRAC) channels that mediate SOCE and evaluated the alkali sensitivity of SOCE in rat odontoblasts. In the absence of extracellular Ca(2+), treatment with thapsigargin (TG), a sarco/endoplasmic reticulum Ca(2+)-ATPase inhibitor, induced an increase in [Ca(2+)]i. After [Ca(2+)]i returned to near-resting levels, the subsequent application of 2.5 mM extracellular Ca(2+) resulted in an increase in [Ca(2+)]i which is a typical of SOCE activation. Additionally, application of 2-Methylthioadenosine diphosphate trisodium salt (2-MeSADP), a P2Y1,12,13 receptor agonist, or carbachol (CCh), a muscarinic cholinergic receptor agonist, in the absence of extracellular Ca(2+), induced a transient increase in [Ca(2+)]i. The subsequent addition of extracellular Ca(2+) resulted in significantly higher [Ca(2+)]i in 2-MeSADP- or CCh-treated odontoblasts than in untreated cells. SOCE, that is activated by addition of extracellular Ca(2+) in the TG pretreated odontoblasts was then suppressed by Synta66, BTP2, or lanthanum, which are CRAC channel inhibitors. Treatment with an alkaline solution enhanced SOCE, while treatment with HC030031, a TRPA1 channel antagonist, inhibited it. The amplitude of SOCE at pH 9 in the presence of HC030031 was higher than that at pH 7.4 in the absence of HC030031. These findings indicate that CRAC channel-mediated alkali-sensitive SOCE occurs in odontoblasts. SOCE is mediated by P2Y and muscarinic-cholinergic receptors, which are activated by endogenous ligands in odontoblasts.
Glycolysis and oxidative phosphorylation are essential for purinergic receptor-mediated angiogenic responses in vasa vasorum endothelial cells.[Pubmed:27856430]
Am J Physiol Cell Physiol. 2017 Jan 1;312(1):C56-C70.
Angiogenesis is an energy-demanding process; however, the role of cellular energy pathways and their regulation by extracellular stimuli, especially extracellular nucleotides, remain largely unexplored. Using metabolic inhibitors of glycolysis (2-deoxyglucose) and oxidative phosphorylation (OXPHOS) (oligomycin, rotenone, and FCCP), we demonstrate that glycolysis and OXPHOS are both essential for angiogenic responses of vasa vasorum endothelial cell (VVEC). Treatment with P2R agonists, ATP, and 2-Methylthioadenosine diphosphate trisodium salt (MeSADP), but not P1 receptor agonist, adenosine, increased glycolytic activity in VVEC (measured by extracellular acidification rate and lactate production). Stimulation of glycolysis was accompanied by increased levels of phospho-phosphofructokinase B3, hexokinase (HK), and GLUT-1, but not lactate dehydrogenase. Moreover, extracellular ATP and MeSADP, and to a lesser extent adenosine, increased basal and maximal oxygen consumption rates in VVEC. These effects were potentiated when the cells were cultured in 20 mM galactose and 5 mM glucose compared with 25 mM glucose. Treatment with P2R agonists decreased phosphorylation of pyruvate dehydrogenase (PDH)-E1alpha and increased succinate dehydrogenase (SDH), cytochrome oxidase IV, and beta-subunit of F1F0 ATP synthase expression. In addition, P2R stimulation transiently elevated mitochondrial Ca(2+) concentration, implying involvement of mitochondria in VVEC angiogenic activation. We also demonstrated a critical role of phosphatidylinositol 3-kinase and Akt pathways in lactate production, PDH-E1alpha phosphorylation, and the expression of HK, SDH, and GLUT-1 in ATP-stimulated VVEC. Together, our findings suggest that purinergic and metabolic regulation of VVEC energy pathways is essential for VV angiogenesis and may contribute to pathologic vascular remodeling in pulmonary hypertension.
A retrospective of recombinant P2Y receptor subtypes and their pharmacology.[Pubmed:11747319]
Arch Biochem Biophys. 2002 Jan 1;397(1):131-6.
Since the first cloning of P2Y receptor sequences in 1993 it has become apparent that this family of G-protein-coupled receptors is omnipresent. At least 25 individual sequences entered in the GenBank sequence database encode P2Y receptors from a variety of species ranging from the little skate Raja erinacea to man. In man, six receptor subtypes have been cloned and found to be functionally active (P2Y(1), P2Y(2), P2Y(4), P2Y(6), P2Y(11), and P2Y(12)). In this article a review of the P2Y receptor subtypes is presented considering both their sequences and the pharmacological profiles of the encoded receptors expressed in heterologous expression systems.
P2Y(13): identification and characterization of a novel Galphai-coupled ADP receptor from human and mouse.[Pubmed:11961076]
J Pharmacol Exp Ther. 2002 May;301(2):705-13.
We have identified an orphan G protein-coupled receptor, SP174, that shares a high degree of homology with the recently described ADP receptor P2Y(12). mRNA for SP174 is abundant in the brain and in cells of the immune system. In the present study, we demonstrate that SP174 is also a receptor for ADP, which is coupled to Galphai. ADP potently stimulates SP174 with an EC(50) of 60 nM, and other related nucleotides are active as well, with a rank order of potency 2-methylthio-ADP tetrasodium = adenosine 5'-O-2-(thio)diphosphate = 2-methylthio-ATP tetrasodium > ADP > AP3A >ATP > IDP. This pharmacological profile is similar to that for P2Y(12). We have also identified the murine homolog of SP174, which exhibits 75% homology to the human receptor. ADP is also a potent agonist at the murine receptor, and its pharmacological profile is similar to its human counterpart, but ADP and related nucleotides are more potent at the murine receptor than the human receptor. In keeping with the general nomenclature for the purinergic receptors, we propose designating this novel receptor P2Y(13).
2-Methylthioadenosine[beta-32P]diphosphate. An agonist and radioligand for the receptor that inhibits the accumulation of cyclic AMP in intact blood platelets.[Pubmed:6298277]
J Clin Invest. 1983 Mar;71(3):420-8.
2-Methylthio-ADP and its radioactive analogue [beta-32P]2-methylthio-ADP were synthesized and used to investigate platelet receptors for ADP. 2-Methylthio-ADP induced platelet aggregation and shape change, and inhibited cyclic AMP accumulation in platelets exposed to prostaglandin E1. Compared with ADP, 2-methylthio-ADP was 3-5 times as active as an aggregating agent and 150-200 times as active as an inhibitor of cyclic AMP accumulation. Binding of [beta-32P]2-methylthio-ADP to platelets was measured after centrifuging them through silicone oil to separate platelets from their suspension medium. Binding was reversible, saturable, and specific, with between 400 and 1,200 sites/cell in different platelet preparations. There was no evidence for a second class of binding sites with different affinity. The second order association rate constant was approximately 3.5 X 10(6) M-1 S-1, and the first order dissociation rate was 0.024 s-1, both measured at 23 degrees C. The dissociation equilibrium constant (approximately 15 nM) was about three times higher than the concentration giving half-maximal inhibition of prostaglandin E1-stimulated cyclic AMP accumulation in platelet-rich plasma. Binding was inhibited by ADP (Ki = 3.5 microM), ATP (7 microM), 2-azido-ADP (0.12 microM), inosine diphosphate (IDP, 150 microM), guanosine diphosphate (GDP, 350 microM), and AMP (800 microM). Binding of 2-methylthio-ADP was also blocked by the non-cell-penetrating thiol reagent, p-mercuribenzene sulphonate, a reagent that blocks the inhibition of adenylate cyclase by ADP, but which does not block the ability of ADP to induce aggregation or platelet shape change. The amount of 2-methylthio-ADP bound at saturation was independent of pH in the range 6-8, but the affinity was reduced at pH 6 compared with pH 6.5-8.0. The dissociation constant was not temperature dependent in the range 32 degrees -40 degrees C, whereas the rate of dissociation of 2-methylthio-ADP from platelets after the addition of an excess of ADP approximately doubled over this range. The activation energy for dissociation was approximately 15 kcal/mol. Our results support the conclusion that platelets have a receptor for ADP, which inhibits cyclic AMP accumulation, and which has a sulphydryl group in the binding pocket.


