Aristolochic acid BCAS# 475-80-9 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 475-80-9 | SDF | Download SDF |
| PubChem ID | 108168 | Appearance | Yellow powder |
| Formula | C16H9NO6 | M.Wt | 311.25 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Aristolochic acid B | ||
| Solubility | Sparingly soluble in methanol; practically insoluble in water | ||
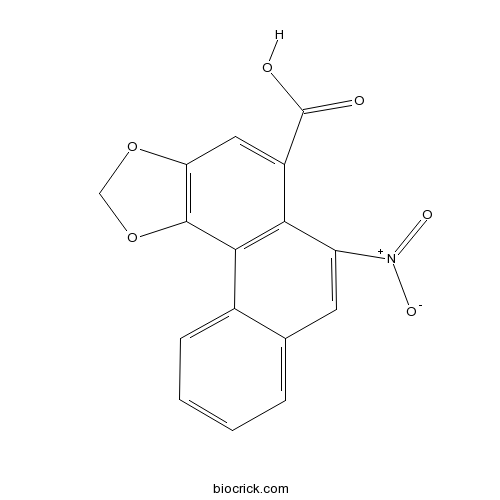
| Chemical Name | 6-nitronaphtho[2,1-g][1,3]benzodioxole-5-carboxylic acid | ||
| SMILES | C1OC2=C(O1)C3=C(C(=C2)C(=O)O)C(=CC4=CC=CC=C43)[N+](=O)[O-] | ||
| Standard InChIKey | MEEXETVZNQYRSP-UHFFFAOYSA-N | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Aristolochic acid II (Aristolochic acid B,AAII), one of the major components of the carcinogenic plant extract aristolochic acid, is known to be mutagenic and to form DNA adducts in vitro and in vivo, AAII shows more carcinogenic risk than aristolochic acid I, and this may be, at least partly, the result of its increased levels in kidney and plasma. |
| In vitro | Production, characterization of a monoclonal antibody against aristolochic acid-B and development of its assay system.[Pubmed: 18457371]Am J Chin Med. 2008;36(2):425-36.Aristolochic acid-II (Aristolochic acid B, AA-II) conjugated with bovine serum albumin (BSA) was used as an antigen for immunizing BALB/c mice.
|
| In vivo | Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney.[Pubmed: 22245565 ]Mutat Res. 2012 Mar 18;743(1-2):52-8.Aristolochic acid (AA) is known to be a potent mutagen and carcinogen. Aristolochic acid I (Aristolochic acid A, AAI) and aristolochic acid II (Aristolochic acid B, AAII), the two major components of AA, differ from each other by a single methoxy group. However, their individual mutagenic characteristics in vivo are unclear.
|
| Kinase Assay | N6-adenyl arylation of DNA by aristolochic acid II and a synthetic model for the putative proximate carcinogen.[Pubmed: 1665354]Chem Res Toxicol. 1991 Sep-Oct;4(5):581-6.Aristolochic acid II (Aristolochic acid B, AAII), one of the major components of the carcinogenic plant extract aristolochic acid, is known to be mutagenic and to form DNA adducts in vitro and in vivo.
|

Aristolochic acid B Dilution Calculator

Aristolochic acid B Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2129 mL | 16.0643 mL | 32.1285 mL | 64.257 mL | 80.3213 mL |
| 5 mM | 0.6426 mL | 3.2129 mL | 6.4257 mL | 12.8514 mL | 16.0643 mL |
| 10 mM | 0.3213 mL | 1.6064 mL | 3.2129 mL | 6.4257 mL | 8.0321 mL |
| 50 mM | 0.0643 mL | 0.3213 mL | 0.6426 mL | 1.2851 mL | 1.6064 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3213 mL | 0.6426 mL | 0.8032 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Liriodenine
Catalog No.:BCN5532
CAS No.:475-75-2
- (+)-Isocorynoline
Catalog No.:BCN2361
CAS No.:475-67-2
- H-N-Me-Pro-OH
Catalog No.:BCC3351
CAS No.:475-11-6
- SB 657510
Catalog No.:BCC7713
CAS No.:474960-44-6
- ST 91
Catalog No.:BCC7436
CAS No.:4749-61-5
- 2,16-Kauranediol 2-O-beta-D-allopyranoside
Catalog No.:BCN1436
CAS No.:474893-07-7
- Monomethyl auristatin E
Catalog No.:BCC1775
CAS No.:474645-27-7
- N-Methylsarpagine methosalt
Catalog No.:BCN5530
CAS No.:47418-70-2
- CAL-130 Racemate
Catalog No.:BCC1442
CAS No.:474012-90-3
- Brassicasterol
Catalog No.:BCN2613
CAS No.:474-67-9
- Campesterol
Catalog No.:BCN3181
CAS No.:474-62-4
- Campestanol
Catalog No.:BCN3890
CAS No.:474-60-2
- Glaucine
Catalog No.:BCN2550
CAS No.:475-81-0
- Nuciferine
Catalog No.:BCN1223
CAS No.:475-83-2
- NS 304
Catalog No.:BCC7661
CAS No.:475086-01-2
- Tivozanib (AV-951)
Catalog No.:BCC1179
CAS No.:475108-18-0
- ZSTK474
Catalog No.:BCC3657
CAS No.:475110-96-4
- Galnon
Catalog No.:BCC5871
CAS No.:475115-35-6
- BAN ORL 24
Catalog No.:BCC1398
CAS No.:475150-69-7
- 2-Methylthioadenosine diphosphate trisodium salt
Catalog No.:BCC5794
CAS No.:475193-31-8
- A-317491
Catalog No.:BCC1320
CAS No.:475205-49-3
- Sorafenib Tosylate
Catalog No.:BCC3654
CAS No.:475207-59-1
- Nogo-66 (1-40)
Catalog No.:BCC5862
CAS No.:475221-20-6
- CORM-3
Catalog No.:BCC5108
CAS No.:475473-26-8
Production, characterization of a monoclonal antibody against aristolochic acid-II and development of its assay system.[Pubmed:18457371]
Am J Chin Med. 2008;36(2):425-36.
Aristolochic acid-II (AA-II) conjugated with bovine serum albumin (BSA) was used as an antigen for immunizing BALB/c mice. Isolated splenocytes from the immunized mice were fused with an aminopterin-sensitive mouse myeloma cell line, SP2/0-Ag14, to produce hybridoma cells that secreted a monoclonal antibody (MAb) against AA-II. The selected hybridoma was subsequently cloned by limited dilution method. For MAb, the isotype and an estimated dissociation constant (K(D)) of the MAb were determined. The MAb was used to establish an ELISA method. Accuracy and variation assays, as well as determinations of the specificity and sensitivity, were also carried out and the linear range was 0.19-13 microg/ml. The anti-AA-II MAb showed a very high specificity for AA-II and had low cross-reactivities against the other aristolochic acid (AAs) (CR: AA-I, 3.4%; AA-VIIa, 0.86%) or aristololactam-I (AL-I) (CR<0.07%) except AA-IIIa which has 17% of cross activity. Anti-AA-II MAb also showed negligible cross-reactivity (<0.5%) toward other natural compounds with different chemical structures including barbaloin, sennoside A, rutin, glycyrrhizin, caffeic acid etc. This is the first time that an ELISA method was successfully established for the application of anti-AA-II MAb.
N6-adenyl arylation of DNA by aristolochic acid II and a synthetic model for the putative proximate carcinogen.[Pubmed:1665354]
Chem Res Toxicol. 1991 Sep-Oct;4(5):581-6.
Aristolochic acid II (AAII), one of the major components of the carcinogenic plant extract aristolochic acid, is known to be mutagenic and to form DNA adducts in vitro and in vivo. The major fluorescent DNA adduct formed upon xanthine oxidase mediated reduction in the presence of calf thymus (CT-) DNA or deoxyadenosine was isolated by means of preparative HPLC and identified by fluorescence, UV/vis absorbance, and 1H NMR spectroscopy as 7-(deoxy-adenosin-N6-yl)aristolactam II. As a model proximate carcinogen, N-chloroaristolactam II was prepared chemically from aristolactam II, the reduction product of AAII. This model compound was spectroscopically characterized and found to react directly with CT-DNA without any activation, forming the same deoxyadenosine adduct. HPLC analysis with fluorescence monitoring detected this adduct in vivo in the liver DNA of Wistar rats treated orally with AAII. These results confirm the anticipated metabolic activation mechanism of AAII as occurring via a cyclic nitrenium ion.
Comparison of the mutagenicity of aristolochic acid I and aristolochic acid II in the gpt delta transgenic mouse kidney.[Pubmed:22245565]
Mutat Res. 2012 Mar 18;743(1-2):52-8.
Aristolochic acid (AA) is known to be a potent mutagen and carcinogen. Aristolochic acid I (AAI) and aristolochic acid II (AAII), the two major components of AA, differ from each other by a single methoxy group. However, their individual mutagenic characteristics in vivo are unclear. In the present study, we compared their DNA adduct formation and mutagenicities in the gpt delta transgenic mouse kidney. The dA-AAI, dG-AAI, dA-AAII and dG-AAII were identified in the kidney two days after intragastric administration of AAI or AAII at 5mg/kg. The concentration of DNA adducts formed by AAII was approximately 2.5-fold higher than that formed by AAI (p<0.05). The mutant frequency induced by AAII was nearly two-fold higher than that induced by AAI (p<0.05) following administration of 5mg/kg AAI or AAII, five times per week for six weeks. Investigation of the mutation spectra showed no statistically significant difference between AAI- and AAII-treated mice (p>0.05). A:T to T:A transversion was the predominant type of mutation in both treated groups, the GC-associated mutation rates, however, differed between the AAI and AAII treatments. The in vivo metabolic pathways of AAI and AAII are different, and this may affect their mutagenicity. In the present study, we measured the levels of AAI and AAII in the kidney and plasma of gpt delta transgenic mice at multiple time points after a single intragastric dose of 1 or 5mg/kg of either component. Our results showed that the levels of AAII in both kidney and plasma were considerably higher than those of AAI (p<0.01). The present study indicated that AAII showed more carcinogenic risk than AAI in vivo, and this may be, at least partly, the result of its increased levels in kidney and plasma.


