Maleic acidCAS# 110-16-7 |
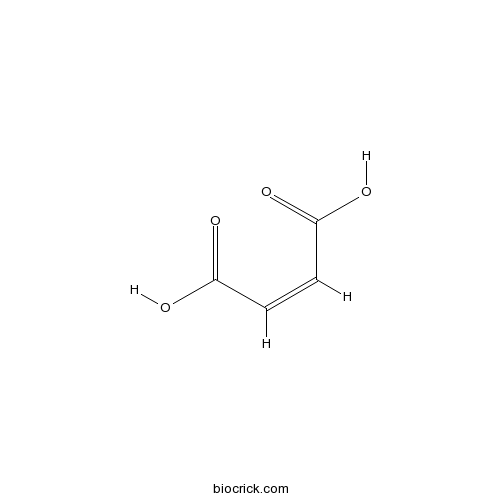
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110-16-7 | SDF | Download SDF |
| PubChem ID | 444266 | Appearance | White powder |
| Formula | C4H4O4 | M.Wt | 116.07 |
| Type of Compound | Organic acids & Esters | Storage | Desiccate at -20°C |
| Synonyms | cis-2-Butenedioic acid | ||
| Solubility | Soluble in ethanol and water | ||
| Chemical Name | (Z)-but-2-enedioic acid | ||
| SMILES | C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | VZCYOOQTPOCHFL-UPHRSURJSA-N | ||
| Standard InChI | InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1- | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Maleic acid Dilution Calculator

Maleic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6155 mL | 43.0775 mL | 86.1549 mL | 172.3098 mL | 215.3873 mL |
| 5 mM | 1.7231 mL | 8.6155 mL | 17.231 mL | 34.462 mL | 43.0775 mL |
| 10 mM | 0.8615 mL | 4.3077 mL | 8.6155 mL | 17.231 mL | 21.5387 mL |
| 50 mM | 0.1723 mL | 0.8615 mL | 1.7231 mL | 3.4462 mL | 4.3077 mL |
| 100 mM | 0.0862 mL | 0.4308 mL | 0.8615 mL | 1.7231 mL | 2.1539 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Succinic acid
Catalog No.:BCN5890
CAS No.:110-15-6
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- Kifunensine
Catalog No.:BCC7601
CAS No.:109944-15-2
- Sodium prasterone sulfate
Catalog No.:BCC9149
CAS No.:1099-87-2
- Granisetron
Catalog No.:BCC1601
CAS No.:109889-09-0
- CGS 9343B
Catalog No.:BCC7303
CAS No.:109826-27-9
- Kaempferol 5,7,4'-trimethyl ether
Catalog No.:BCN6587
CAS No.:1098-92-6
- Harmalidine
Catalog No.:BCN5889
CAS No.:109794-97-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- 8 beta-(4-Acetoxy-5-hydroxytigloyloxy)costunolide
Catalog No.:BCN7123
CAS No.:109770-86-7
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
Enhanced stability of L-asparaginase by its bioconjugation to poly(styrene-co-maleic acid) and Ecoflex nanoparticles.[Pubmed:29768231]
IET Nanobiotechnol. 2018 Jun;12(4):466-472.
Acute lymphoblastic leukemia (ALL) is the white blood cell cancer in children. L-asparaginase (L-ASNase) is one of the first drugs used in ALL treatment. Anti-tumor activity of L-ASNase is not specific and indicates limited stability in different biological environments, in addition to its quick clearance from blood. The purpose of the present study was to achieve a new L-ASNase polymer bioconjugate to improve pharmacokinetic, increase half-life and stability of the enzyme. The conjugations were achieved by the cross-linking agent of 1-ethyl-3-(3- dimethylaminopropyl) carbodiimide (EDC) which activates the carboxylic acid groups of polymeric nanoparticles to create amide bond. EDC conjugated the L-ASNase to two biodegradable polymers including; Ecoflex((R)) and poly (styrene-co-Maleic acid) (PSMA) nanoparticles. To achieve optimal L-ASNase nanoparticles the amounts of each polymer and the crosslinker were optimized and the nanoparticles were characterized according to their particle size, zeta potential and percent of conjugation of the enzyme. The results showed that conjugated enzyme had more stability against pH changes and proteolysis. It had lower Km value (indicating more affinity to the substrate) and greater half-life in plasma and phosphate buffered saline, in comparison to native enzyme. Generally, the conjugated enzyme to PSMA nanoparticles showed greater results than Ecoflex((R)) nanoparticles.
Structure of the alternative complex III in a supercomplex with cytochrome oxidase.[Pubmed:29695868]
Nature. 2018 May;557(7703):123-126.
Alternative complex III (ACIII) is a key component of the respiratory and/or photosynthetic electron transport chains of many bacteria(1-3). Like complex III (also known as the bc1 complex), ACIII catalyses the oxidation of membrane-bound quinol and the reduction of cytochrome c or an equivalent electron carrier. However, the two complexes have no structural similarity(4-7). Although ACIII has eluded structural characterization, several of its subunits are known to be homologous to members of the complex iron-sulfur molybdoenzyme (CISM) superfamily (8) , including the proton pump polysulfide reductase(9,10). We isolated the ACIII from Flavobacterium johnsoniae with native lipids using styrene Maleic acid copolymer(11-14), both as an independent enzyme and as a functional 1:1 supercomplex with an aa3-type cytochrome c oxidase (cyt aa3). We determined the structure of ACIII to 3.4 A resolution by cryo-electron microscopy and constructed an atomic model for its six subunits. The structure, which contains a [3Fe-4S] cluster, a [4Fe-4S] cluster and six haem c units, shows that ACIII uses known elements from other electron transport complexes arranged in a previously unknown manner. Modelling of the cyt aa3 component of the supercomplex revealed that it is structurally modified to facilitate association with ACIII, illustrating the importance of the supercomplex in this electron transport chain. The structure also resolves two of the subunits of ACIII that are anchored to the lipid bilayer with N-terminal triacylated cysteine residues, an important post-translational modification found in numerous prokaryotic membrane proteins that has not previously been observed structurally in a lipid bilayer.
Salt formation improved the properties of a candidate drug during early formulation development.[Pubmed:29730322]
Eur J Pharm Sci. 2018 Jul 30;120:162-171.
The purpose of this study was to investigate if AZD5329, a dual neurokinin NK1/2 receptor antagonist, is a suitable candidate for further development as an oral immediate release (IR) solid dosage form as a final product. The neutral form of AZD5329 has only been isolated as amorphous material. In order to search for a solid material with improved physical and chemical stability and more suitable solid-state properties, a salt screen was performed. Crystalline material of a Maleic acid salt and a fumaric acid salt of AZD5329 were obtained. X-ray powder diffractiometry, thermogravimetric analysis, differential scanning calorimetry and dynamic vapor sorption were used to investigate the physicochemical characteristics of the two salts. The fumarate salt of AZD5329 is anhydrous, the crystallization is reproducible and the hygroscopicity is acceptable. Early polymorphism assessment work using slurry technique did not reveal any better crystal modification or crystallinity for the fumarate salt. For the maleate salt, the form isolated originally was found to be a solvate, but an anhydrous form was found in later experiments; by suspension in water or acetone, by drying of the solvate to 100-120 degrees C or by subjecting the solvate form to conditions of 40 degrees C/75%RH for 3months. The dissolution behavior and the chemical stability (in aqueous solutions, formulations and solid-state) of both salts were also studied and found to be satisfactory. The compound displays sensitivity to low pH, and the salt of the Maleic acid, which is the stronger acid, shows more degradation during stability studies, in line with this observation. The presented data indicate that the substance fulfils basic requirements for further development of an IR dosage form, based on the characterization on crystalline salts of AZD5329.
Production of Plant Phthalate and its Hydrogenated Derivative from Bio-Based Platform Chemicals.[Pubmed:29624916]
ChemSusChem. 2018 May 25;11(10):1621-1627.
Direct transformation of bio-based platform chemicals into aromatic dicarboxylic acids and their derivatives, which are widely used for the manufacture of polymers, is of significant importance for the sustainable development of the plastics industry. However, limited successful chemical processes have been reported. This study concerns a sustainable route for the production of phthalate and its hydrogenated derivative from bio-based malic acid and erythritol. The key Diels-Alder reaction is applied to build a substituted cyclohexene structure. The dehydration reaction of malic acid affords fumaric acid with 96.6 % yield, which could be used as the dienophile, and 1,3-butadiene generated in situ through erythritol deoxydehydration serves as the diene. Starting from erythritol and dibutyl fumarate, a 74.3 % yield of dibutyl trans-4-cyclohexene-1,2-dicarboxylate is obtained. The palladium-catalyzed dehydrogenation of the cycloadduct gives a 77.8 % yield of dibutyl phthalate. Dibutyl trans-cyclohexane-1,2-dicarboxylate could be formed in nearly 100 % yield under mild conditions by hydrogenation of the cycloadduct. Furthermore, fumaric acid and fumarate, with trans configurations, were found to be better dienophiles for this Diels-Alder reaction than Maleic acid and maleate, with cis configuration, based on the experimental and computational results. This new route will pave the way for the production of environmental friendly plastic materials from plants.
Taste characteristics of Chinese bayberry juice characterized by sensory evaluation, chromatography analysis, and an electronic tongue.[Pubmed:29666514]
J Food Sci Technol. 2018 May;55(5):1624-1631.
To evaluate the taste characteristics of Chinese bayberry juice, four types of bayberry juice sourced from different origins and varieties were analysed using sensory evaluation, chromatography, spectroscopy analysis and an electronic tongue (E-tongue). Nine organic acids and three sugars were assessed using high performance liquid chromatography. Total polyphenols were measured by spectrophotometry. The overall taste profile was collected using the E-tongue. The four types of bayberry juice differed in the sensory attributes of sour, sweet, bitter, and astringent. The E-tongue responses combined with discriminant analysis were able to characterise the taste profiles of the juices. The relationships between the taste compounds and the sensory panel scores established by partial least squares showed that total polyphenols, quininic acid, Maleic acid, fructose, citric acid, lactic acid, succinic acid and sucrose made significant contributions to the taste characteristics of the Chinese bayberry juice.
Lignin-based hydrogels with "super-swelling" capacities for dye removal.[Pubmed:29655884]
Int J Biol Macromol. 2018 Aug;115:1249-1259.
Lignin is a complex natural polymer and it is one of the main constituent of the lignocellulosic biomass. Moreover, it is a bio-renewable material and it is available in large amounts as by-product from the forest industry. Lignin-based hydrogels with high swelling capabilities were prepared by crosslinking poly (methyl vinyl ether co-Maleic acid) and different technical lignins in ammonium and sodium hydroxide solutions. The produced hydrogels showed a wide range of water absorption capacities varying from 13 to 130g of water per 1g of sample. It was observed that the higher the water uptake the poorer mechanical performance, as evaluated in terms of storage and loss modulus (G' and G'', respectively) of the materials. Methylene blue (MB) was used as a model dye to evaluate the adsorption and release capabilities of the lignin hydrogels. Results suggested that these hydrogels showed a high MB removal efficiency, which ranged from 12 to 96%. On the contrary, the percentages of MB released depended on the negative surface charge of the hydrogels, showing values which ranged from 0.06 to 0.35%. Thus, these materials have potential to be used as adsorbents for the removal of organic dyes from waste water.
Microwave-Assisted Oxalic Acid Pretreatment for the Enhancing of Enzyme Hydrolysis in the Production of Xylose and Arabinose from Bagasse.[Pubmed:29642578]
Molecules. 2018 Apr 10;23(4). pii: molecules23040862.
In this study, highly-efficient hydrolysis of bagasse into xylose and arabinose sugars (C5 sugars) was developed by microwave-assisted oxalic acid pretreatment under mild reaction conditions. The effects of acid and hydrolysis conditions on the C5 sugar yields were discussed. The results showed that oxalic acid performed better than hydrochloric acid and Maleic acid, and was a promising alternative to sulfuric acid for xylose production at the same acid concentration. The maximum yields of xylose (95.7%) and arabinose (91.5%) were achieved via the microwave-assisted oxalic acid pretreatment (120 degrees C, 10 min, 0.4 mol/L, solid-liquid ratio of 1:50 g/mL), indicating that almost all xylan-type hemicelluloses were released from the cell wall and hydrolyzed into C5 sugars. After pretreatment, more than 90% of the cellulose in the residual bagasse was converted to glucose (92.2%) by enzymatic hydrolysis. This approach could realize the highly-efficient hydrolysis of xylan from bagasse into C5 sugars, which would enhance the enzyme hydrolysis of treated bagasse into glucose.


