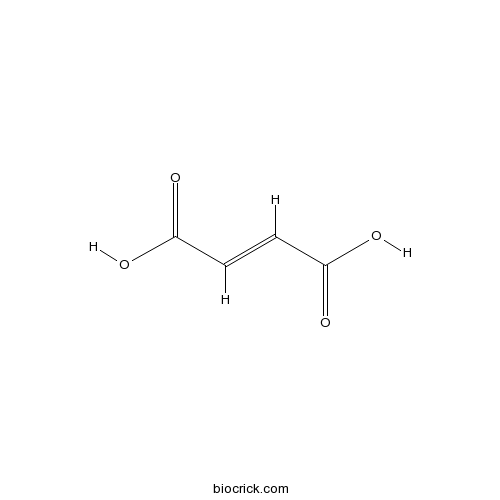
Fumaric acidCAS# 110-17-8 |
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110-17-8 | SDF | Download SDF |
| PubChem ID | 444972 | Appearance | White powder |
| Formula | C4H4O4 | M.Wt | 116.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Synonyms | Allomaleic acid; trans-2-Butenedioic acid; Lichenic acid | ||
| Solubility | Soluble in ethanol; slightly soluble in acetone and water | ||
| Chemical Name | (E)-but-2-enedioic acid | ||
| SMILES | C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | VZCYOOQTPOCHFL-OWOJBTEDSA-N | ||
| Standard InChI | InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fumaric acid is an intermediate in the citric acid cycle used by cells to produce energy in the form of adenosine triphosphate (ATP) from food; also a product of the urea cycle. Fumaric acid is used in systemic and topical treatment of psoriasis. Fumaric acid attenuates the eotaxin-1 expression in TNF-α-stimulated fibroblasts by suppressing p38 MAPK-dependent NF-κB signaling. |
| Targets | TNF-α | NF-kB | p38MAPK |
| In vitro | Metabolic engineering of Escherichia coli for the production of fumaric acid.[Pubmed: 23436277]Biotechnol Bioeng. 2013 Jul;110(7):2025-34.
|
| Kinase Assay | Fumaric acid attenuates the eotaxin-1 expression in TNF-α-stimulated fibroblasts by suppressing p38 MAPK-dependent NF-κB signaling.[Pubmed: 23707484]Food Chem Toxicol. 2013 Aug;58:423-31.In this study, we investigated the effects of Fumaric acid on eotaxin-1 expression in a mouse fibroblast cell line. |
| Cell Research | Fumaric acid production by Torulopsis glabrata: engineering the urea cycle and the purine nucleotide cycle.[Pubmed: 25060134]Biotechnol Bioeng. 2015 Jan;112(1):156-67.A multi-vitamin auxotrophic Torulopsis glabrata strain, a pyruvate producer, was further engineered to produce Fumaric acid. |

Fumaric acid Dilution Calculator

Fumaric acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.6133 mL | 43.0663 mL | 86.1326 mL | 172.2653 mL | 215.3316 mL |
| 5 mM | 1.7227 mL | 8.6133 mL | 17.2265 mL | 34.4531 mL | 43.0663 mL |
| 10 mM | 0.8613 mL | 4.3066 mL | 8.6133 mL | 17.2265 mL | 21.5332 mL |
| 50 mM | 0.1723 mL | 0.8613 mL | 1.7227 mL | 3.4453 mL | 4.3066 mL |
| 100 mM | 0.0861 mL | 0.4307 mL | 0.8613 mL | 1.7227 mL | 2.1533 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
- Succinic acid
Catalog No.:BCN5890
CAS No.:110-15-6
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- Kifunensine
Catalog No.:BCC7601
CAS No.:109944-15-2
- Sodium prasterone sulfate
Catalog No.:BCC9149
CAS No.:1099-87-2
- Granisetron
Catalog No.:BCC1601
CAS No.:109889-09-0
- CGS 9343B
Catalog No.:BCC7303
CAS No.:109826-27-9
- Kaempferol 5,7,4'-trimethyl ether
Catalog No.:BCN6587
CAS No.:1098-92-6
- Harmalidine
Catalog No.:BCN5889
CAS No.:109794-97-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
- Ascomycin
Catalog No.:BCN8286
CAS No.:11011-38-4
- Indoximod (NLG-8189)
Catalog No.:BCC5584
CAS No.:110117-83-4
Metabolic engineering of Escherichia coli for the production of fumaric acid.[Pubmed:23436277]
Biotechnol Bioeng. 2013 Jul;110(7):2025-34.
Fumaric acid is a naturally occurring organic acid that is an intermediate of the tricarboxylic acid cycle. Fungal species belonging to Rhizopus have traditionally been employed for the production of Fumaric acid. In this study, Escherichia coli was metabolically engineered for the production of Fumaric acid under aerobic condition. For the aerobic production of Fumaric acid, the iclR gene was deleted to redirect the carbon flux through the glyoxylate shunt. In addition, the fumA, fumB, and fumC genes were also deleted to enhance Fumaric acid formation. The resulting strain was able to produce 1.45 g/L of Fumaric acid from 15 g/L of glucose in flask culture. Based on in silico flux response analysis, this base strain was further engineered by plasmid-based overexpression of the native ppc gene, encoding phosphoenolpyruvate carboxylase (PPC), from the strong tac promoter, which resulted in the production of 4.09 g/L of Fumaric acid. Additionally, the arcA and ptsG genes were deleted to reinforce the oxidative TCA cycle flux, and the aspA gene was deleted to block the conversion of Fumaric acid into L-aspartic acid. Since it is desirable to avoid the use of inducer, the lacI gene was also deleted. To increase glucose uptake rate and Fumaric acid productivity, the native promoter of the galP gene was replaced with the strong trc promoter. Fed-batch culture of the final strain CWF812 allowed production of 28.2 g/L Fumaric acid in 63 h with the overall yield and productivity of 0.389 g Fumaric acid/g glucose and 0.448 g/L/h, respectively. This study demonstrates the possibility for the efficient production of Fumaric acid by metabolically engineered E. coli.
Fumaric acid attenuates the eotaxin-1 expression in TNF-alpha-stimulated fibroblasts by suppressing p38 MAPK-dependent NF-kappaB signaling.[Pubmed:23707484]
Food Chem Toxicol. 2013 Aug;58:423-31.
Eotaxin-1 is a potent chemoattractant for eosinophils and a critical mediator during the development of eosinophilic inflammation. Fumaric acid is an intermediate product of the citric acid cycle, which is source of intracellular energy. Although Fumaric acid ameliorates psoriasis and multiple sclerosis, its involvement in eotaxin-1-mediated effects has not been assessed. In this study, we investigated the effects of Fumaric acid on eotaxin-1 expression in a mouse fibroblast cell line. We found that Fumaric acid significantly inhibited tumor necrosis factor-alpha (TNF-alpha-induced eotaxin-1 expression. This Fumaric acid effect was mediated through the inhibition of p38 mitogen-activated protein kinase (MAPK)-dependent nuclear factor (NF)-kappaB signaling. We also found that Fumaric acid operates downstream of MEKK3 during TNF-alpha-induced NF-kappaB signaling, which upregulated eotaxin-1 expression. In addition, Fumaric acid attenuated expression of CC-chemokine receptor 3 (CCR3), an eotaxin-1 receptor, and adhesion molecules that play important roles in eosinophil binding to induce allergic inflammation. Taken together, these findings indicate that inhibiting TNF-alpha-induced eotaxin-1 expression by Fumaric acid occurs primarily through suppression of NF-kappaB signaling, which is mediated by inhibiting p38 MAPK and suggest that Fumaric acid may be used as a complementary treatment option for eotaxin-1-mediated diseases.
Fumaric acid production by Torulopsis glabrata: engineering the urea cycle and the purine nucleotide cycle.[Pubmed:25060134]
Biotechnol Bioeng. 2015 Jan;112(1):156-67.
A multi-vitamin auxotrophic Torulopsis glabrata strain, a pyruvate producer, was further engineered to produce Fumaric acid. Using the genome-scale metabolic model iNX804 of T. glabrata, four Fumaric acid biosynthetic pathways, involving the four cytosolic enzymes, argininosuccinate lyase (ASL), adenylosuccinate lyase (ADSL), fumarylacetoacetase (FAA), and fumarase (FUM1), were found. Athough single overexpression of each of the four enzymes in the cytosol improved Fumaric acid production, the highest Fumaric acid titer (5.62 g L(-1) ) was obtained with strain T.G-ASL(H) -ADSL(L) by controlling the strength of ASL at a high level and ADSL at a low level. In order to further improve the production of Fumaric acid, the SpMAE1 gene encoding the C4 -dicarboxylic acids transporter was overexpressed in strain T.G-ASL(H) -ADSL(L) -SpMAE1 and the final Fumaric acid titer increased to 8.83 g L(-1) . This study provides a novel strategy for Fumaric acid biosynthesis by utilizing the urea cycle and the purine nucleotide cycle to enhance the bridge between carbon metabolism and nitrogen metabolism.


