Succinic acidCAS# 110-15-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 110-15-6 | SDF | Download SDF |
| PubChem ID | 1110 | Appearance | Powder |
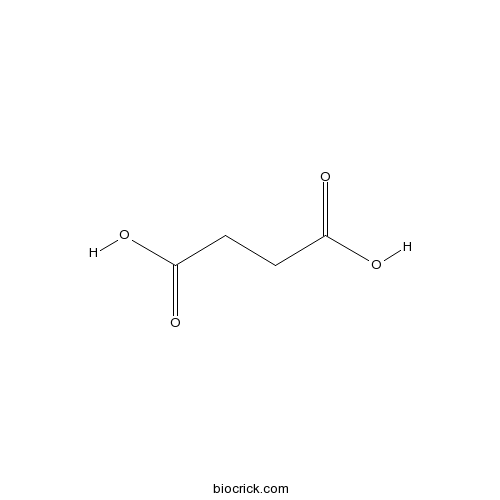
| Formula | C4H6O4 | M.Wt | 118.1 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | DMSO : 100 mg/mL (846.81 mM; Need ultrasonic) H2O : 30 mg/mL (254.04 mM; Need ultrasonic) | ||
| Chemical Name | butanedioic acid | ||
| SMILES | C(CC(=O)O)C(=O)O | ||
| Standard InChIKey | KDYFGRWQOYBRFD-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Succinic acid is a crystalline organic acid which occurs in living tissue as an intermediate in glucose metabolism. |
| Structure Identification | Bioresour Technol. 2015 Feb 26;185C:56-61.Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli.[Pubmed: 25747879]In this study, microbial production of Succinic acid from macroalgae (i.e., Laminaria japonica) was investigated for the first time. The engineered Escherichia coli BS002 exhibited higher molar yield of Succinic acid on mannitol (1.39±0.01mol/mol) than glucose (1.01±0.05mol/mol). After pretreatment and enzymatic hydrolysis, L. japonica hydrolysate was mainly glucose (10.31±0.32g/L) and mannitol (10.12±0.17g/L), which was used as the substrate for Succinic acid fermentation with the recombinant BS002. A final 17.44±0.54g/L Succinic acid was obtained from the hydrolysate after 72h dual-phase fermentation. The yield was as high as 1.24±0.08mol/mol total sugar, which reached 73% of the maximum theoretical yield. |

Succinic acid Dilution Calculator

Succinic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.4674 mL | 42.337 mL | 84.674 mL | 169.348 mL | 211.685 mL |
| 5 mM | 1.6935 mL | 8.4674 mL | 16.9348 mL | 33.8696 mL | 42.337 mL |
| 10 mM | 0.8467 mL | 4.2337 mL | 8.4674 mL | 16.9348 mL | 21.1685 mL |
| 50 mM | 0.1693 mL | 0.8467 mL | 1.6935 mL | 3.387 mL | 4.2337 mL |
| 100 mM | 0.0847 mL | 0.4234 mL | 0.8467 mL | 1.6935 mL | 2.1169 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ITD 1
Catalog No.:BCC6409
CAS No.:1099644-42-4
- Kifunensine
Catalog No.:BCC7601
CAS No.:109944-15-2
- Sodium prasterone sulfate
Catalog No.:BCC9149
CAS No.:1099-87-2
- Granisetron
Catalog No.:BCC1601
CAS No.:109889-09-0
- CGS 9343B
Catalog No.:BCC7303
CAS No.:109826-27-9
- Kaempferol 5,7,4'-trimethyl ether
Catalog No.:BCN6587
CAS No.:1098-92-6
- Harmalidine
Catalog No.:BCN5889
CAS No.:109794-97-0
- ASP3026
Catalog No.:BCC1372
CAS No.:1097917-15-1
- 9R-10alpha-Hydroxyepigambogic acid
Catalog No.:BCN3079
CAS No.:1097882-33-1
- Homopahutoxin
Catalog No.:BCN1812
CAS No.:109777-68-6
- 8 beta-(4-Acetoxy-5-hydroxytigloyloxy)costunolide
Catalog No.:BCN7123
CAS No.:109770-86-7
- cis-Dehydroosthol
Catalog No.:BCN4735
CAS No.:109741-40-4
- Maleic acid
Catalog No.:BCN8426
CAS No.:110-16-7
- Fumaric acid
Catalog No.:BCN5989
CAS No.:110-17-8
- Sorbic acid
Catalog No.:BCN2218
CAS No.:110-44-1
- 8-O-Ethylyunaconitine
Catalog No.:BCN6260
CAS No.:110011-77-3
- Taurohyodeoxycholic Acid Sodium Salt
Catalog No.:BCC8363
CAS No.:110026-03-4
- Tussilagonone
Catalog No.:BCC8365
CAS No.:110042-38-1
- Calpain Inhibitor I, ALLN
Catalog No.:BCC1233
CAS No.:110044-82-1
- Strophantin K (mixture)
Catalog No.:BCC8256
CAS No.:11005-63-3
- 7-Hydroxy-3-(4-hydroxybenzylidene)chroman-4-one
Catalog No.:BCN1624
CAS No.:110064-50-1
- 12-Epinapelline
Catalog No.:BCN2800
CAS No.:110064-71-6
- Plerixafor (AMD3100)
Catalog No.:BCC1158
CAS No.:110078-46-1
- des-His1-[Glu9]-Glucagon (1-29) amide
Catalog No.:BCC5885
CAS No.:110084-95-2
Efficient production of succinic acid from macroalgae hydrolysate by metabolically engineered Escherichia coli.[Pubmed:25747879]
Bioresour Technol. 2015 Jun;185:56-61.
In this study, microbial production of Succinic acid from macroalgae (i.e., Laminaria japonica) was investigated for the first time. The engineered Escherichia coli BS002 exhibited higher molar yield of Succinic acid on mannitol (1.39+/-0.01mol/mol) than glucose (1.01+/-0.05mol/mol). After pretreatment and enzymatic hydrolysis, L. japonica hydrolysate was mainly glucose (10.31+/-0.32g/L) and mannitol (10.12+/-0.17g/L), which was used as the substrate for Succinic acid fermentation with the recombinant BS002. A final 17.44+/-0.54g/L Succinic acid was obtained from the hydrolysate after 72h dual-phase fermentation. The yield was as high as 1.24+/-0.08mol/mol total sugar, which reached 73% of the maximum theoretical yield. The results demonstrate that macroalgae biomass represents a novelty and economical alternative feedstock for biochemicals production.
Capture of carbon dioxide from ethanol fermentation by liquid absorption for use in biological production of succinic acid.[Pubmed:25448631]
Appl Biochem Biotechnol. 2015 Feb;175(4):2104-13.
Previously, it was shown that the gas produced in an ethanol fermentor using either corn or barley as feedstock could be sparged directly into an adjacent fermentor as a feedstock for Succinic acid fermentation using Escherichia coli AFP184. In the present investigation, it was demonstrated that the CO2 produced in a corn ethanol fermentor could be absorbed in a base solution and the resultant carbonate solution used both for pH control and supply of the CO2 requirement in Succinic acid fermentation. Thus, the CO2 produced in a 5-L corn mash containing 30 wt% total solids was absorbed in a packed column containing 2 L of either 5 M NaOH, 5 M KOH, or 15 wt% NH4OH, and the resultant carbonate solutions were used for pH control in a Succinic acid fermentor. The results obtained indicated no significant differences between Succinic acid production in these experiments and when 2.5 M solutions of Na2CO3, K2CO3, and (NH4)2CO3 from commercial sources were used. In a commercial setting, the demonstrated capture of CO2 in liquid form will allow transportation of the carbonate solutions to locations not in the immediate vicinity of the ethanol plant, and excess carbonate salts can also be recovered as value-added products.


