Cediranib (AZD217)VEGFR inhibitor receptor,highly potent CAS# 288383-20-0 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 288383-20-0 | SDF | Download SDF |
| PubChem ID | 9933475 | Appearance | Powder |
| Formula | C25H27FN4O3 | M.Wt | 450.51 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | AZD2171 | ||
| Solubility | DMSO : ≥ 49 mg/mL (108.77 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
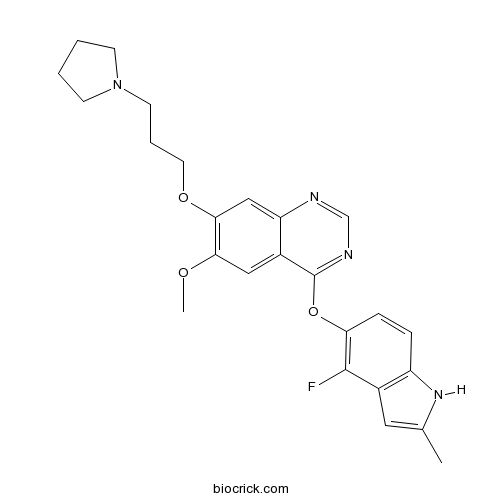
| Chemical Name | 4-[(4-fluoro-2-methyl-1H-indol-5-yl)oxy]-6-methoxy-7-(3-pyrrolidin-1-ylpropoxy)quinazoline | ||
| SMILES | CC1=CC2=C(N1)C=CC(=C2F)OC3=NC=NC4=CC(=C(C=C43)OC)OCCCN5CCCC5 | ||
| Standard InChIKey | XXJWYDDUDKYVKI-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C25H27FN4O3/c1-16-12-17-19(29-16)6-7-21(24(17)26)33-25-18-13-22(31-2)23(14-20(18)27-15-28-25)32-11-5-10-30-8-3-4-9-30/h6-7,12-15,29H,3-5,8-11H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cediranib is a highly potent inhibitor of VEGFR with IC50 value of <1 nM. | |||||
| Targets | VEGFR2/KDR | c-Kit | VEGFR3/FLT4 | VEGFR1/FLT1 | PDGFRβ | FGFR1 |
| IC50 | 0.5 nM | 2 nM | ≤3 nM | 5 nM | 5 nM | 26 nM |

Cediranib (AZD217) Dilution Calculator

Cediranib (AZD217) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2197 mL | 11.0985 mL | 22.1971 mL | 44.3941 mL | 55.4927 mL |
| 5 mM | 0.4439 mL | 2.2197 mL | 4.4394 mL | 8.8788 mL | 11.0985 mL |
| 10 mM | 0.222 mL | 1.1099 mL | 2.2197 mL | 4.4394 mL | 5.5493 mL |
| 50 mM | 0.0444 mL | 0.222 mL | 0.4439 mL | 0.8879 mL | 1.1099 mL |
| 100 mM | 0.0222 mL | 0.111 mL | 0.222 mL | 0.4439 mL | 0.5549 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Cediranib (also known as AZD2171) is a highly potent KDR tyrosine kinase inhibitor that ATP-competitively inhibits recombinant KDR tyrosine kinase as well as other members of vascular endothelial growth factor receptor (VEGFR) family, including Flt-1 (VEGFR-1) and Flt-4 (VEGFR-3) with the half maximal inhibition concentration IC50 values of <0.001 μmol/L, 0.005 μmol/L and <0.003 μmol/L respectively [1].
Cediranib also potently inhibits a few members of platelet-derived growth factor receptor (PDGFR) family, including c-Kit, PDGFR-β, PDGFR-α, CSF-1R and Flt-3, with IC50 values of 0.002 μmol/L, 0.005 μmol/L, 0.036 μmol/L, 0.11 μmol/L anf > 1 μmol/L respectively due to their similar structure to VEGFR family members [1].
References:
[1] Wedge SR, Kendrew J, Hennequin LF, Valentine PJ, Barry ST, Brave SR, Smith NR, James NH, Dukes M, Curwen JO, Chester R, Jackson JA, Boffey SJ, Kilburn LL, Barnett S, Richmond GH, Wadsworth PF, Walker M, Bigley AL, Taylor ST, Cooper L, Beck S, Jürgensmeier JM, Ogilvie DJ. AZD2171: a highly potent, orally bioavailable, vascular endothelial growth factor receptor-2 tyrosine kinase inhibitor for the treatment of cancer. Cancer Res. 2005 May 15;65(10):4389-400.
- (-)-dicentrine
Catalog No.:BCC8167
CAS No.:28832-07-7
- Lithospermic acid
Catalog No.:BCN5369
CAS No.:28831-65-4
- alpha-Asarone
Catalog No.:BCN3837
CAS No.:2883-98-9
- Denudanolide A
Catalog No.:BCN6522
CAS No.:288259-72-3
- Y-320
Catalog No.:BCC5202
CAS No.:288250-47-5
- Phlorigidoside B
Catalog No.:BCN5189
CAS No.:288248-46-4
- 3,4-Dihydroxyphenylglycol
Catalog No.:BCN5188
CAS No.:28822-73-3
- IBMX
Catalog No.:BCC7502
CAS No.:28822-58-4
- Z-Ala-OMe
Catalog No.:BCC3056
CAS No.:28819-05-8
- SB408124
Catalog No.:BCC4972
CAS No.:288150-92-5
- Cixiophiopogon A
Catalog No.:BCN2778
CAS No.:288143-27-1
- Fraxinellone
Catalog No.:BCN1272
CAS No.:28808-62-0
- H-Asp(OBzl)-OBzl.TosOH
Catalog No.:BCC2886
CAS No.:2886-33 -1
- S-(-)-Carbidopa
Catalog No.:BCN8453
CAS No.:28860-95-9
- Z-D-Met-OH
Catalog No.:BCC2758
CAS No.:28862-80-8
- Ergosta-5,24(28)-diene-3,7,16-triol
Catalog No.:BCN5190
CAS No.:289054-34-8
- 3,7,16-Trihydroxystigmast-5-ene
Catalog No.:BCN5191
CAS No.:289056-24-2
- Triazolam
Catalog No.:BCC5218
CAS No.:28911-01-5
- Fmoc-Cl
Catalog No.:BCC2802
CAS No.:28920-43-6
- Anodendrine
Catalog No.:BCN1956
CAS No.:28942-07-6
- Canertinib dihydrochloride
Catalog No.:BCC1449
CAS No.:289499-45-2
- Allo-Thr-OH
Catalog No.:BCC3101
CAS No.:28954-12-3
- Cassiachromone
Catalog No.:BCN5192
CAS No.:28955-30-8
- Oridonin
Catalog No.:BCN5953
CAS No.:28957-04-2
Efficacy of targeted therapy for metastatic renal cell carcinoma in the elderly patient population.[Pubmed:24819320]
Clin Genitourin Cancer. 2014 Oct;12(5):354-8.
INTRODUCTION/BACKGROUND: Targeted therapy has become the mainstay of treatment for mRCC. The efficacy of this therapy in the older population is poorly understood. PATIENTS AND METHODS: Data from patients with mRCC treated with first-line anti-VEGF therapy were collected through the International mRCC Database Consortium from 12 centers. Patient characteristics, data on second-line therapy, and outcomes including treatment duration and overall survival, were evaluated using summary statistics and multivariate analysis. RESULTS: All patients (n = 1381) were treated with front-line targeted therapy; 144 (10%) were 75 years old or older. Six patients (4%) were favorable risk, 99 patients (69%) intermediate risk, and 39 patients (27%) poor risk according to Heng Journal of Clinical Oncology 2009 prognostic factors. The initial treatment for those >/= 75 years of age was sunitinib (n = 98), sorafenib (n = 35), bevacizumab (n = 7), and AZD217 (n = 4). Twenty-three percent of older patients and 39% of the younger patients went on to receive second-line therapy (P < .0001). The overall response rate, median treatment duration, and overall survival for the older versus younger group were 18% versus 25% (P = .0975), 5.5 months versus 7.5 months (P = .1388), and 16.8 months versus 19.7 months (P = .3321), respectively. When adjusted for poor prognostic factors, age 75 years and older was not found to be associated with poorer overall survival (hazard ratio [HR], 1.002; 95% confidence interval [CI], 0.781-1.285) or shorter treatment duration (HR, 1.018; 95% CI, 0.827-1.252). The retrospective study design was the primary limitation. CONCLUSION: The use of advanced age as a selection criterion for targeted therapy requires further study, with data suggesting no clinically meaningful differences in overall response rate, treatment duration, and overall survival between older and younger age groups.


