AlvimopanMu-opioid receptors antagonist CAS# 156053-89-3 |
- Alvimopan monohydrate
Catalog No.:BCC1349
CAS No.:1383577-62-5
- Alvimopan dihydrate
Catalog No.:BCC1348
CAS No.:170098-38-1
- JDTic
Catalog No.:BCC1670
CAS No.:361444-66-8
- ADL5859 HCl
Catalog No.:BCC1265
CAS No.:850173-95-4
- Cebranopadol
Catalog No.:BCC1467
CAS No.:863513-91-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156053-89-3 | SDF | Download SDF |
| PubChem ID | 5488548 | Appearance | Powder |
| Formula | C25H32N2O4 | M.Wt | 424.53 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | ADL 8-2698; LY 246736;Alvimopan anhydrous | ||
| Solubility | DMSO | ||
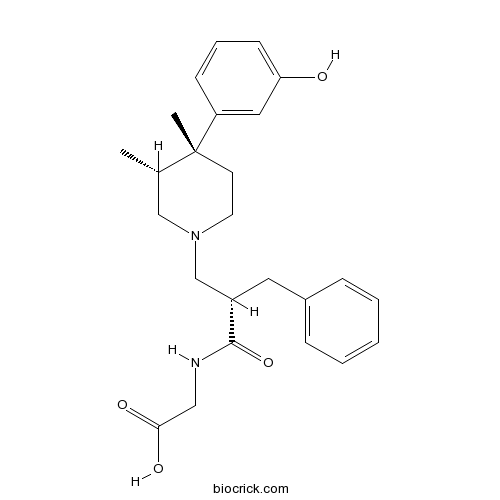
| Chemical Name | 2-[[(2S)-2-benzyl-3-[(3R,4R)-4-(3-hydroxyphenyl)-3,4-dimethylpiperidin-1-yl]propanoyl]amino]acetic acid | ||
| SMILES | CC1CN(CCC1(C)C2=CC(=CC=C2)O)CC(CC3=CC=CC=C3)C(=O)NCC(=O)O | ||
| Standard InChIKey | UPNUIXSCZBYVBB-JVFUWBCBSA-N | ||
| Standard InChI | InChI=1S/C25H32N2O4/c1-18-16-27(12-11-25(18,2)21-9-6-10-22(28)14-21)17-20(24(31)26-15-23(29)30)13-19-7-4-3-5-8-19/h3-10,14,18,20,28H,11-13,15-17H2,1-2H3,(H,26,31)(H,29,30)/t18-,20-,25+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Alvimopan(LY 246736; ADL 8-2698) is a peripherally acting mu-opioid receptor (PAM-OR, IC50= 1.7 nM) antagonist for accelerating gastrointestinal recovery after surgery.
IC50 Value: 1.7 nM (Mu-type opioid receptor) [1]
Target: mu-opioid receptor
in vitro: The dissociation rate of alvimopan from the micro opioid receptor (t(1/2)=30--44 min) was comparable to that of the long acting partial agonist buprenorphine (t(1/2)=44 min), but was slower than those of the antagonists naloxone (t(1/2)=0.82 min) and N-methylnaltrexone (t(1/2)=0.46 min) [2].
in vivo: Alvimopan did not significantly accelerate GI-3 compared with placebo [6 mg: hazard ratio (HR) = 1.20, p = 0.080; 12 mg: HR = 1.24, p = 0.038). However, after adjustment for significant covariates (sex/surgical duration), benefits were significant for both doses (6 mg: HR = 1.24, p = 0.037; 12 mg: HR = 1.26, p = 0.028). Alvimopan also significantly accelerated time to GI-2 (6 mg: HR = 1.37, p = 0.008; 12 mg: HR = 1.33, p = 0.018) and DCO (6 mg: HR = 1.31, p = 0.008; 12 mg: HR = 1.28, p = 0.015) [3]. Alvimopan (1 and 3 mg/kg) significantly reversed this delayed GI transit when administered 45 min prior to surgery. However, the effects of alvimopan were less pronounced when administered following surgery [4].
Toxicity:The most common treatment-emergent adverse events across all treatment groups were nausea, vomiting, and hypotension; the incidence of nausea and vomiting was reduced by 53 percent in thealvimopan 12-mg group [5].
Clinical trial: Intercostal Nerve Block With Liposome Bupivacaine in Subjects Undergoing Posterolateral Thoracotomy. Phase 3 References: | |||||

Alvimopan Dilution Calculator

Alvimopan Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3555 mL | 11.7777 mL | 23.5555 mL | 47.1109 mL | 58.8887 mL |
| 5 mM | 0.4711 mL | 2.3555 mL | 4.7111 mL | 9.4222 mL | 11.7777 mL |
| 10 mM | 0.2356 mL | 1.1778 mL | 2.3555 mL | 4.7111 mL | 5.8889 mL |
| 50 mM | 0.0471 mL | 0.2356 mL | 0.4711 mL | 0.9422 mL | 1.1778 mL |
| 100 mM | 0.0236 mL | 0.1178 mL | 0.2356 mL | 0.4711 mL | 0.5889 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Alvimopan(ADL 8-2698; Entereg; LY 246736) is a selective and competitive antagonist at mu-opioid receptors, found in myenteric and submucosal neurons and the immune cells of the lamina propria in the human gut. Upon administration, Alvimopan(ADL 8-2698; Entereg; LY 246736) binds to mu-opioid receptors in the gut, thereby reversing opiod-related disturbances in gut motility. Alvimopan is approximately three to nine times more potent than naloxone.A synthetic trans-3,4-dimethyl-4-(3-hydroxyphenyl) piperidine with peripherally selective opioid mu receptor antagonist activity.
- Qianhucoumarin E
Catalog No.:BCN3506
CAS No.:156041-02-0
- Angelidiol
Catalog No.:BCN7964
CAS No.:156009-77-7
- H-HoArg-OH
Catalog No.:BCC3226
CAS No.:156-86-5
- Sodium butyrate
Catalog No.:BCC4720
CAS No.:156-54-7
- 4-Hydroxyphenylacetic acid
Catalog No.:BCN4795
CAS No.:156-38-7
- Rabdoternin F
Catalog No.:BCN6399
CAS No.:155977-87-0
- Edultin
Catalog No.:BCC8321
CAS No.:15591-75-0
- Gipsoside
Catalog No.:BCN8482
CAS No.:15588-68-8
- LE 135
Catalog No.:BCC7242
CAS No.:155877-83-1
- 9,10-Dimethoxycanthin-6-one
Catalog No.:BCN3106
CAS No.:155861-51-1
- RJR-2403
Catalog No.:BCC1901
CAS No.:15585-43-0
- BW 373U86
Catalog No.:BCC5798
CAS No.:155836-50-3
- SGC 0946
Catalog No.:BCC2216
CAS No.:1561178-17-3
- Lys-γ3-MSH
Catalog No.:BCC6049
CAS No.:156159-18-1
- BQ-788 sodium salt
Catalog No.:BCC5151
CAS No.:156161-89-6
- Maackiaflavanone
Catalog No.:BCN6834
CAS No.:156162-10-6
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- Hispidulin 7-O-neohesperidoside
Catalog No.:BCN2952
CAS No.:156186-00-4
- GTS 21 dihydrochloride
Catalog No.:BCC7948
CAS No.:156223-05-1
- PyAOP
Catalog No.:BCC2819
CAS No.:156311-83-0
- 11-Anhydro-16-oxoalisol A
Catalog No.:BCN7703
CAS No.:156338-93-1
- Ehretioside B
Catalog No.:BCN1703
CAS No.:156368-84-2
- Ailanthoidol
Catalog No.:BCN7705
CAS No.:156398-61-7
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
Is there value in alvimopan in minimally invasive colorectal surgery?[Pubmed:27262754]
Am J Surg. 2016 Nov;212(5):851-856.
BACKGROUND: Alvimopan's goal is to minimize postoperative ileus and optimize outcomes; however, evidence in laparoscopic surgery is lacking. Our goal was to evaluate the benefit of Alvimopan in laparoscopic colorectal surgery with an enhanced recovery pathway (ERP). METHODS: Laparoscopic colorectal cases were stratified into Alvimopan and control cohorts, then case-matched for comparability. All followed an identical ERP. The main outcomes were length of stay, complications, readmissions, and costs in the Alvimopan and control groups. RESULTS: About 321 patients were analyzed in each cohort. Operative times were comparable (P = .08). Postoperatively, complication rates were similar (P = .29), with no difference in ileus (P = 1.00). The length of stay (3.69 vs 3.49 days; P = .16), readmission (2.8% vs 3.7%; P = .66) and reoperation rates (2.2% vs 1.6%; P = .77) were comparable for Alvimopan and controls, respectively. Total costs were similar ($14,932.47 Alvimopan vs $14,846.56 controls; P = .90), but the additional costs in the Alvimopan group could translate to savings of $27,577 in the cohort. CONCLUSIONS: Alvimopan added no benefit in patient outcomes in laparoscopic colorectal surgery with an ERP. These results could drive a change in current practice. Controlled studies are warranted to define the cost and/or benefit in clinical practice.
Redefining the implications of nasogastric tube placement following radical cystectomy in the alvimopan era.[Pubmed:27476163]
World J Urol. 2017 Apr;35(4):625-631.
PURPOSE: Alvimopan has decreased ileus and need for nasogastric tube (NGT) after radical cystectomy (RC). However, the natural history of ileus versus intestinal obstruction in patients receiving Alvimopan is not well defined. We sought to examine the implications of NGT placement before and after the introduction of Alvimopan for RC patients. METHODS: Retrospective review identified 278 and 293 consecutive patients who underwent RC before and after instituting Alvimopan between June 2009 and May 2014. Baseline characteristics and postoperative outcomes were compared by Alvimopan status. Multivariate logistic regression was performed to assess the impact of Alvimopan on rates of NGT placement and reoperation for bowel complications. RESULTS: The cohorts had similar age, stage, approach, and BMI. Patients receiving Alvimopan had decreased ileus (16 vs 32 %, p < 0.01) but similar rates of reoperation for bowel complications (2.8 vs 2.7 %). On multivariate analysis, Alvimopan was associated with lower risk of NGT placement (OR 0.30, p < 0.01). For patients requiring NGT placement, there was an increased rate of reoperation among patients receiving Alvimopan compared with those who did not (28 vs 11 %, p = 0.03). Patients receiving Alvimopan who needed NGT had significantly increased median length of stay (22 vs 7 days), need for TPN (66 vs 5.3 %), and readmission for ileus (10.3 vs 2.3 %) compared with those who did not require NGT. CONCLUSIONS: Alvimopan significantly reduced the incidence of ileus and NGT placement following RC. NGT placement was associated with an increased need for reoperation for bowel complications in the setting of Alvimopan.
Alvimopan in the setting of colorectal resection with an ostomy: To use or not to use?[Pubmed:27928668]
Surg Endosc. 2017 Sep;31(9):3483-3488.
BACKGROUND: Postoperative ileus (POI) is a major cause of morbidity, increased length of stay (LOS) and hospital cost after colorectal surgery. Alvimopan is a micro-opioid antagonist used to accelerate upper and lower gastrointestinal function after bowel resection. We hypothesized that Alvimopan would reduce LOS in patients undergoing colorectal resection with stoma, a situation that has not been evaluated. METHODS: A retrospective review (2010-2015) identified 58 patients who underwent colorectal resection for benign or malignant disease with stoma creation and received Alvimopan. They were case-matched to 58 non-Alvimopan patients based on age, BMI, baseline comorbidities, stoma type created and surgical approach. We compared overall LOS, incidence of POI and other postoperative complications. RESULTS: There were equal numbers of laparoscopic (N = 18) and open resections (N = 40) in the Alvimopan group and non-Alvimopan group. There were also equal numbers of patients with an ileostomy (N = 37) or colostomy (N = 21) in each group. Overall, 41 patients underwent resection for malignant disease in the Alvimopan group compared to 37 in the non-Alvimopan group. There was a significant reduction in median LOS overall (Alvimopan 5 (4-7) versus control 6 (4.75-9.25) days, P = 0.03). While the 6-day median LOS was similar for patients undergoing ileostomy creation (P = 0.25), Alvimopan patients had a 3-day decreased median LOS that approached statistical significance (P = 0.06). The overall 30-day complication rate was higher in the control group (41.4 vs. 51.7%, P = 0.26), but the readmission rate within 30 days was higher in the Alvimopan group (19 vs. 13.8%, P = 0.45). Neither of these differences reached statistically significance. CONCLUSION: The use of Alvimopan in patients undergoing colorectal resection with stoma is associated with a significantly shorter LOS, but the increased readmission rate warrants further study. Based on these data, Alvimopan should be evaluated in a controlled setting for patients undergoing colorectal resection with colostomy creation.
Alvimopan for post-operative ileus: What we should know?[Pubmed:27825721]
Acta Anaesthesiol Taiwan. 2016 Sep;54(3):97-98.
Alvimopan is an US-FDA approved, peripherally acting mu opioid receptor antagonist which when started pre-operatively has been shown to hasten intestinal motility and reduce the duration of post-operative ileus. However the logistics involved in procuring, storing and dispensing the drug and the cost of the drug for fifteen doses as approved by FDA prohibits the use of it on a regular basis.


