GTS 21 dihydrochloridenAChRs agonist, novel CAS# 156223-05-1 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Cadherin Peptide, avian
Catalog No.:BCC1018
CAS No.:127650-08-2
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 156223-05-1 | SDF | Download SDF |
| PubChem ID | 6438361 | Appearance | Powder |
| Formula | C19H22Cl2N2O2 | M.Wt | 381.3 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | DMXBA | ||
| Solubility | Soluble to 50 mM in DMSO | ||
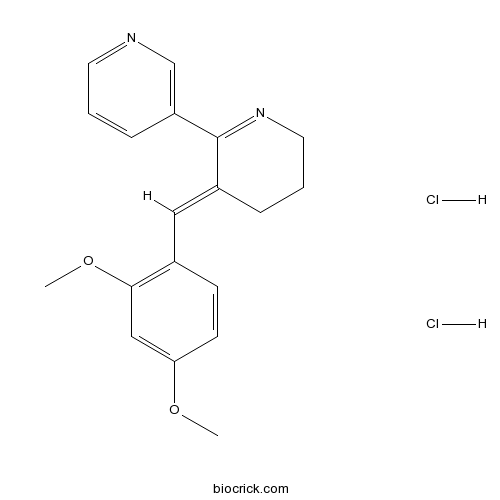
| Chemical Name | 3-[(2,4-Dimethoxyphenyl)methylene]- | ||
| SMILES | [H+].[H+].[Cl-].[Cl-].COc1ccc(C=C2/CCCN=C2c3cccnc3)c(OC)c1 | ||
| Standard InChIKey | BXKYFUGAAFLYJL-BXGYHSFXSA-N | ||
| Standard InChI | InChI=1S/C19H20N2O2.2ClH/c1-22-17-8-7-14(18(12-17)23-2)11-15-5-4-10-21-19(15)16-6-3-9-20-13-16;;/h3,6-9,11-13H,4-5,10H2,1-2H3;2*1H/b15-11+;; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Partial agonist of α7 nicotinic acetylcholine receptors (nAChRs); also a weak α4β2 and 5-HT3 antagonist at micromolar concentrations. Exhibits improved sensory inhibition in DBA/2 mice following both acute and chronic administration. Also shown to improve memory in several animal models; facilitates hippocampal long-term potentiation. |

GTS 21 dihydrochloride Dilution Calculator

GTS 21 dihydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6226 mL | 13.113 mL | 26.2261 mL | 52.4521 mL | 65.5652 mL |
| 5 mM | 0.5245 mL | 2.6226 mL | 5.2452 mL | 10.4904 mL | 13.113 mL |
| 10 mM | 0.2623 mL | 1.3113 mL | 2.6226 mL | 5.2452 mL | 6.5565 mL |
| 50 mM | 0.0525 mL | 0.2623 mL | 0.5245 mL | 1.049 mL | 1.3113 mL |
| 100 mM | 0.0262 mL | 0.1311 mL | 0.2623 mL | 0.5245 mL | 0.6557 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
GTS 21 dihydrochloride is a novel agonist of nicotinic acetylcholine receptors (nAChRs) [1].
nAChRs are neuron receptor proteins that activated by the binding of the neurotransmitter acetylcholine (ACh).
In RAW 264.7 cells (a macrophage like cell line) exposed to hyperoxia (≥99% O2), GTS-21 significantly increased phagocytic activity of macrophages in a dose-dependent way and reduced hyperoxia-induced hyperacetylation of HMGB1. Also, GTS-21 inhibited the cytoplasmic translocation and release of HMGB1 from these macrophages [1]. GTS-21 bound to human α4β2 nAChR (Ki value of 20 nM) 100-fold more potently than to human α7 nAChR, and was 2- and 18-fold less potent than (2)-nicotine at human α7 and α4β2 nAChR, respectively [2].
In mice that were exposed to hyperoxia (≥99% O2) and subsequently challenged with PA, intraperitoneal injection of GTS-21 (4 mg/kg) significantly increased bacterial clearance, decreased accumulation of airway HMGB1 and decreased acute lung injury [1]. GTS-21 stimulated dopamine release from rat striatal slices with an EC50 of 10uM. In the delayed matching-to-sample task, GTS-21 (32–130 nmol/kg) improved learning performance of monkeys [2].
References:
[1]. Sitapara RA, Antoine DJ, Sharma L, et al. The α7 nicotinic acetylcholine receptor agonist GTS-21 improves bacterial clearance in mice by restoring hyperoxia-compromised macrophage function. Mol Med, 2014, 20: 238-247.
[2]. Briggs CA, Anderson DJ, Brioni JD, et al. Functional characterization of the novel neuronal nicotinic acetylcholine receptor ligand GTS-21 in vitro and in vivo. Pharmacol Biochem Behav, 1997, 57(1-2): 231-241.
- Hispidulin 7-O-neohesperidoside
Catalog No.:BCN2952
CAS No.:156186-00-4
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- Maackiaflavanone
Catalog No.:BCN6834
CAS No.:156162-10-6
- BQ-788 sodium salt
Catalog No.:BCC5151
CAS No.:156161-89-6
- Lys-γ3-MSH
Catalog No.:BCC6049
CAS No.:156159-18-1
- SGC 0946
Catalog No.:BCC2216
CAS No.:1561178-17-3
- Alvimopan
Catalog No.:BCC1347
CAS No.:156053-89-3
- Qianhucoumarin E
Catalog No.:BCN3506
CAS No.:156041-02-0
- Angelidiol
Catalog No.:BCN7964
CAS No.:156009-77-7
- H-HoArg-OH
Catalog No.:BCC3226
CAS No.:156-86-5
- Sodium butyrate
Catalog No.:BCC4720
CAS No.:156-54-7
- 4-Hydroxyphenylacetic acid
Catalog No.:BCN4795
CAS No.:156-38-7
- PyAOP
Catalog No.:BCC2819
CAS No.:156311-83-0
- 11-Anhydro-16-oxoalisol A
Catalog No.:BCN7703
CAS No.:156338-93-1
- Ehretioside B
Catalog No.:BCN1703
CAS No.:156368-84-2
- Ailanthoidol
Catalog No.:BCN7705
CAS No.:156398-61-7
- 3,4-Seco-3-oxobisabol-10-ene-4,1-olide
Catalog No.:BCN7550
CAS No.:1564265-85-5
- CWHM-12
Catalog No.:BCC5548
CAS No.:1564286-55-0
- cis-Khellactone
Catalog No.:BCN3703
CAS No.:15645-11-1
- α-Conotoxin ImI
Catalog No.:BCC5974
CAS No.:156467-85-5
- Myricetin 3-O-galactoside
Catalog No.:BCN4703
CAS No.:15648-86-9
- 1-Hydroxytropacocaine
Catalog No.:BCN1919
CAS No.:156497-23-3
- 7,13-Dideacetyl-9,10-didebenzoyltaxchinin C
Catalog No.:BCN7670
CAS No.:156497-25-5
- Ryanodine
Catalog No.:BCC5742
CAS No.:15662-33-6
Hydroxy metabolites of the Alzheimer's drug candidate 3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (GTS-21): their molecular properties, interactions with brain nicotinic receptors, and brain penetration.[Pubmed:14722237]
Mol Pharmacol. 2004 Jan;65(1):56-67.
3-[(2,4-dimethoxy)benzylidene]-anabaseine dihydrochloride (DMXBA; GTS-21), an Alzheimer's drug candidate, selectively stimulates alpha7 nicotinic acetylcholine receptors. It rapidly enters the brain after oral administration and enhances cognitive behavior. Less than 1% of orally administered DMXBA is recovered in the urine. We report the identification and characterization of the major phase I metabolites of this drug candidate. Three hydroxy metabolites were generated in vitro by hepatic microsomal O-dealkylation of the two methoxy substituents on the benzylidene ring. They were also found in plasma of rats after oral administration, but at significantly lower concentrations relative to the parent compound. The metabolites displayed similar binding affinities and partial agonist potencies at rat brain alpha7 receptors. However, each displayed a higher efficacy than DMXBA for stimulating rat and human alpha7 receptors. Like DMXBA, the metabolites were weak antagonists at alpha4beta2 receptors. The predicted conformations of the metabolites were nearly identical with that of DMXBA. Ionization of the tetrahydropyridyl nitrogen was essential for high-affinity binding of DMXBA to the alpha7 receptor. The hydroxy metabolites were much more polar than DMXBA, derived from their experimentally estimated octanol/water partition coefficients, and they entered the brain much less readily than DMXBA. Their contributions to the behavioral effects of orally administered DMXBA, if any, would probably be very small during short-term administration. Benzylidene anabaseines pharmacologically similar to the hydroxy metabolites, but which enter the brain more readily, may provide greater stimulation of alpha7 receptors in the whole organism.
Nicotinic mechanisms in the treatment of psychotic disorders: a focus on the alpha7 nicotinic receptor.[Pubmed:23027417]
Handb Exp Pharmacol. 2012;(213):211-32.
Nicotine is heavily abused by persons with schizophrenia. Nicotine better enables people with schizophrenia to filter out extraneous auditory stimuli. Nicotine also improves prepulse inhibition when compared to placebo. Nicotine similarly increases the amplitude of patients' duration mismatch negativity. The 15q13-14 region of the genome coding for the alpha7 nicotinic receptor is linked to schizophrenia. Multiple single nucleotide polymorphisms have been identified in this 15q13-14 gene promoter region that are more frequently present in people with schizophrenia than in normal controls. Abnormalities in expression and regulation of central nicotinic cholinoceptors with decreased alpha7 binding in multiple brain regions are also present. Nicotine enhances cognition in schizophrenia. Alternative agents that activate the nicotinic receptor have been tested including 3-[2,4-dimethoxybenzylidene]anabaseine (DMXB-A). This compound improved attention, working memory, and negative symptoms in an add-on study in nonsmoking patients with schizophrenia. There are multiple other nicotinic agents, including positive allosteric modulators, in the preclinical stages of development. Finally, the effects of varenicline and clozapine and their relation to smoking cessation are discussed.
Continuous administration of a selective alpha7 nicotinic partial agonist, DMXBA, improves sensory inhibition without causing tachyphylaxis or receptor upregulation in DBA/2 mice.[Pubmed:20599427]
Brain Res. 2010 Sep 17;1352:140-6.
Stimulation of nicotinic receptors, specifically the alpha7 subtype, improves sensory inhibition and cognitive function in receptor deficient humans and rodents. However, stimulation with a full agonist, such as nicotine, produces rapid tachyphylaxis of the P20N40-measured sensory inhibition process. 3-(2,4-dimethoxybenzylidine) anabaseine (DMXBA, also GTS-21) selectively activates the alpha7 nicotinic receptor, and in acute administration studies, has been shown to improve deficient sensory inhibition in both humans and rodents with repeated dosing. Unlike nicotine, this partial agonist acted without inducing tachyphylaxis. Here, we assessed the ability of DMXBA to improve sensory inhibition in DBA/2 mice after 7 days of continuous administration via a subcutaneously implanted osmotic minipump. When assessed on day 8, mice receiving saline showed the characteristic deficient sensory inhibition seen with untreated DBA/2 mice. The 25- and 50-mg/ml infusion concentrations of DMXBA, but not the 100-mg/ml, produced significantly improved sensory inhibition in the mice, exclusively through a decrease in test amplitude. No concentration significantly upregulated hippocampal alpha7 receptor levels. DMXBA levels in the brain were higher than plasma at 2 of the 3 concentrations infused. These data suggest that continuous exposure to DMXBA does not significantly affect the underlying responsiveness of the sensory inhibition pathway to this partial agonist, nor cause receptor upregulation, at these relatively low brain concentrations. The ability of DMXBA to maintain its effectiveness during constant administration conditions may be due to an ability to activate alpha7 receptors at low concentrations, and consequently low fractional occupancy of the five possible binding sites on this homomeric receptor.


