FraxinelloneCAS# 28808-62-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28808-62-0 | SDF | Download SDF |
| PubChem ID | 124039 | Appearance | White powder |
| Formula | C14H16O3 | M.Wt | 232.28 |
| Type of Compound | Sesquiterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in acetone and methan | ||
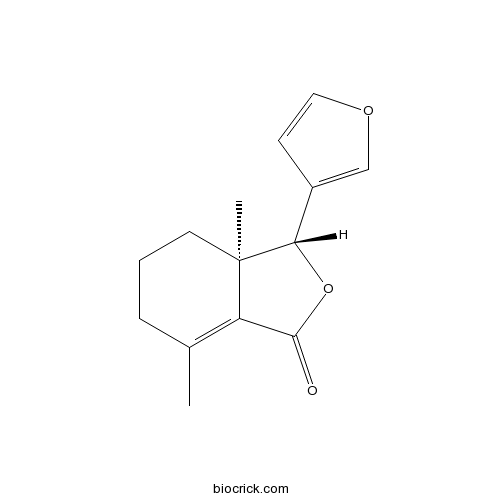
| Chemical Name | (3R,3aR)-3-(furan-3-yl)-3a,7-dimethyl-3,4,5,6-tetrahydro-2-benzofuran-1-one | ||
| SMILES | CC1=C2C(=O)OC(C2(CCC1)C)C3=COC=C3 | ||
| Standard InChIKey | XYYAFLHHHZVPRN-GXTWGEPZSA-N | ||
| Standard InChI | InChI=1S/C14H16O3/c1-9-4-3-6-14(2)11(9)13(15)17-12(14)10-5-7-16-8-10/h5,7-8,12H,3-4,6H2,1-2H3/t12-,14+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Fraxinellone is a selective blocker of voltage-dependent Ca2+ channel, which possesses antimicrobial, anti-inflammatory, neuroprotective and vasorelaxing activities, Fraxinellone exhibits a variety of insecticidal activities including feeding-deterrent activity, inhibition of growth, and larvicidal activity. It inhibited the production of iNOS, COX-2, NF-kappa B, and PGE(2). |
| Targets | NOS | IL Receptor | gp120/CD4 | COX | NF-kB | IkB | ERK | Calcium Channel | NO | JNK | PGE | p65 | p38MAPK | Bcl-2/Bax | Antifection | IKK |
| In vitro | Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice.[Pubmed: 19428326 ]Biochem Pharmacol. 2009 Jun 1;77(11):1717-24.Selectively inducing apoptosis of activated T cells is essential for the clearance of pathogenic injurious cells and subsequent efficient resolution of inflammation. However, few chemicals have been reported to trigger apoptosis of activated T cells in the treatment of hepatitis without affecting quiescent T cells.
Insecticidal and feeding deterrent effects of fraxinellone from Dictamnus dasycarpus against four major pests.[Pubmed: 23455666]Molecules. 2013 Mar 1;18(3):2754-62.Fraxinellone, a well-known and significant naturally occurring compound isolated from Meliaceae and Rutaceae spp. has been widely used as a drug for the treatment of tumors. On the other hand, Fraxinellone exhibited a variety of insecticidal activities including feeding-deterrent activity, inhibition of growth, and larvicidal activity.
|
| In vivo | Suppression of NF-κB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone.[Pubmed: 25448682]Toxicol Appl Pharmacol. 2014 Oct 13;281(1):146-156.Inflammatory bowel disease (IBD) affects millions of people worldwide. Although the etiology of this disease is uncertain, accumulating evidence indicates a key role for the activated mucosal immune system. In the present study, we examined the effects of the natural compound Fraxinellone on dextran sulfate sodium (DSS)-induced colitis in mice, an animal model that mimics IBD.
|
| Kinase Assay | Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers.[Pubmed: 1377790]Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):349-55.The components of Dictamnus dasycarpus Turcz were tested for their vasorelaxing effect on the rat aorta, and Fraxinellone and dictamine were shown to be effective vasorelaxants.
|
| Cell Research | Fraxinellone inhibits lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by negatively regulating nuclear factor-kappa B in RAW 264.7 macrophages cells.[Pubmed: 19483316]Biol Pharm Bull. 2009 Jun;32(6):1062-8.Fraxinellone is formed by the natural degradation of limonoids isolated from the root bark of Dictamnus dasycarpus. Fraxinellone has been reported to possess neuroprotective and vasorelaxing activities, but the effects and the mechanism of Fraxinellone in inflammation have not been fully characterized.
|

Fraxinellone Dilution Calculator

Fraxinellone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3051 mL | 21.5257 mL | 43.0515 mL | 86.103 mL | 107.6287 mL |
| 5 mM | 0.861 mL | 4.3051 mL | 8.6103 mL | 17.2206 mL | 21.5257 mL |
| 10 mM | 0.4305 mL | 2.1526 mL | 4.3051 mL | 8.6103 mL | 10.7629 mL |
| 50 mM | 0.0861 mL | 0.4305 mL | 0.861 mL | 1.7221 mL | 2.1526 mL |
| 100 mM | 0.0431 mL | 0.2153 mL | 0.4305 mL | 0.861 mL | 1.0763 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 4,7-Bis(5-bromo-2-thienyl)-2,1,3-benzothiadiazole
Catalog No.:BCC8668
CAS No.:288071-87-4
- Heraclenin
Catalog No.:BCN5187
CAS No.:2880-49-1
- Tetrazole
Catalog No.:BCC2847
CAS No.:288-94-8
- Peptone, bacteriological
Catalog No.:BCC1210
CAS No.:288-88-0
- H-Cys(Acm)-OH.HCl
Catalog No.:BCC2903
CAS No.:28798-28-9
- Nordihydrocapsaicin
Catalog No.:BCN2387
CAS No.:28789-35-7
- 4,5,6,7-Tetrahydrothieno [3,2,c]pyridine hydrochloride
Catalog No.:BCC8664
CAS No.:28783-41-7
- Rosuvastatin
Catalog No.:BCC4139
CAS No.:287714-41-4
- Apigenin 5-O-beta-D-glucopyranoside
Catalog No.:BCN5185
CAS No.:28757-27-9
- 3CAI
Catalog No.:BCC5402
CAS No.:28755-03-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Oxcarbazepine
Catalog No.:BCC5077
CAS No.:28721-07-5
- Cixiophiopogon A
Catalog No.:BCN2778
CAS No.:288143-27-1
- SB408124
Catalog No.:BCC4972
CAS No.:288150-92-5
- Z-Ala-OMe
Catalog No.:BCC3056
CAS No.:28819-05-8
- IBMX
Catalog No.:BCC7502
CAS No.:28822-58-4
- 3,4-Dihydroxyphenylglycol
Catalog No.:BCN5188
CAS No.:28822-73-3
- Phlorigidoside B
Catalog No.:BCN5189
CAS No.:288248-46-4
- Y-320
Catalog No.:BCC5202
CAS No.:288250-47-5
- Denudanolide A
Catalog No.:BCN6522
CAS No.:288259-72-3
- alpha-Asarone
Catalog No.:BCN3837
CAS No.:2883-98-9
- Lithospermic acid
Catalog No.:BCN5369
CAS No.:28831-65-4
- (-)-dicentrine
Catalog No.:BCC8167
CAS No.:28832-07-7
- Cediranib (AZD217)
Catalog No.:BCC1121
CAS No.:288383-20-0
Insecticidal and feeding deterrent effects of fraxinellone from Dictamnus dasycarpus against four major pests.[Pubmed:23455666]
Molecules. 2013 Mar 1;18(3):2754-62.
Fraxinellone, a well-known and significant naturally occurring compound isolated from Meliaceae and Rutaceae spp. has been widely used as a drug for the treatment of tumors. On the other hand, Fraxinellone exhibited a variety of insecticidal activities including feeding-deterrent activity, inhibition of growth, and larvicidal activity. The present study focused on the antifeedant and larvicidal activities of Fraxinellone against the larvae of Lepidoptera, including Mythimna separata, Agrotis ypsilon, Plutella xylostella, and one kind of sanitary pest, Culux pipiens pallens. Meanwhile, the ovicidal activities and the effects of Fraxinellone on the larval development of M. separata were also observed. The LC50 values of Fraxinellone against 3rd instar larvae of M. separata, 2nd instar larvae of P. xylostella and 4th instar larvae of C. pipiens pallens were 15.95/6.43/3.60 x 10-2 mg mL-1, and its AFC50 values against 5th instar larvae of M. separata, 2nd instar larvae of P. xylostella and 2nd instar larvae of A. ypsilon were 10.73/7.93/12.58 mg mL-1, respectively. Compared with the control group, Fraxinellone obviously inhibited the pupation rate and the growth of M. separata. Once M. separata was treated with Fraxinellone at concentrations of 5.0, 10.0, and 20.0 mg mL-1, respectively, the stages from the larvae to adulthood and the egg hatching duration were prolonged to 1/2/3, and 4/3/4 days, respectively. Additionally, Fraxinellone strongly inhibited the development rate and the egg hatch proportion of M. separata.
Fraxinellone inhibits lipopolysaccharide-induced inducible nitric oxide synthase and cyclooxygenase-2 expression by negatively regulating nuclear factor-kappa B in RAW 264.7 macrophages cells.[Pubmed:19483316]
Biol Pharm Bull. 2009 Jun;32(6):1062-8.
Fraxinellone is formed by the natural degradation of limonoids isolated from the root bark of Dictamnus dasycarpus. Fraxinellone has been reported to possess neuroprotective and vasorelaxing activities, but the effects and the mechanism of Fraxinellone in inflammation have not been fully characterized. In the present study, the anti-inflammatory effect of Fraxinellone was evaluated in lipopolysaccharide (LPS)-treated RAW 264.7 macrophages. Fraxinellone was found to inhibit LPS-induced nitric oxide (NO) and prostaglandin E(2) (PGE(2)) production, and to reduce the LPS-induced expressions of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) at the mRNA and protein levels in a dose-dependent manner. Furthermore, Fraxinellone significantly attenuated LPS-induced DNA binding activity and the transcription activity of nuclear factor-kappa B (NF-kappaB). Consistent with these findings, pretreatment with Fraxinellone significantly suppressed the LPS-stimulated phosphorylation of inhibitory kappa B-alpha (IkappaB-alpha) and the subsequent translocation of p65 to the nucleus. Fraxinellone also suppressed the IkappaB kinase (IKK) activity and the phosphorylation of extracellular-signal-related kinase (ERK1/2), whereas the phosphorylations of Jun N-terminal kinase (JNK1/2) and p38 were unaffected. These results suggest that the anti-inflammatory properties of Fraxinellone are related to the down-regulations of iNOS and COX-2 due to NF-kappaB inhibition through the negative regulations of IKK and ERK1/2 phosphorylations in RAW 264.7 cells.
Suppression of NF-kappaB signaling and NLRP3 inflammasome activation in macrophages is responsible for the amelioration of experimental murine colitis by the natural compound fraxinellone.[Pubmed:25448682]
Toxicol Appl Pharmacol. 2014 Nov 15;281(1):146-56.
Inflammatory bowel disease (IBD) affects millions of people worldwide. Although the etiology of this disease is uncertain, accumulating evidence indicates a key role for the activated mucosal immune system. In the present study, we examined the effects of the natural compound Fraxinellone on dextran sulfate sodium (DSS)-induced colitis in mice, an animal model that mimics IBD. Treatment with Fraxinellone significantly reduced weight loss and diarrhea in mice and alleviated the macroscopic and microscopic signs of the disease. In addition, the activities of myeloperoxidase and alkaline phosphatase were markedly suppressed, while the levels of glutathione were increased in colitis tissues following Fraxinellone treatment. This compound also decreased the colonic levels of interleukin (IL)-1beta, IL-6, IL-18 and tumor necrosis factor (TNF)-alpha in a concentration-dependent manner. These effects of Fraxinellone in mice with experimental colitis were attributed to its inhibition of CD11b(+) macrophage infiltration. The mRNA levels of macrophage-related molecules in the colon, including intercellular adhesion molecule 1 (ICAM1), vascular cell adhesion molecule 1 (VCAM1), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX2), were also markedly inhibited following Fraxinellone treatment. The results from in vitro assays showed that Fraxinellone significantly reduced lipopolysaccharide (LPS)-induced production of nitric oxide (NO), IL-1beta and IL-18 as well as the activity of iNOS in both THP-1 cells and mouse primary peritoneal macrophages. The mechanisms responsible for these effects were attributed to the inhibitory role of Fraxinellone in NF-kappaB signaling and NLRP3 inflammasome activation. Overall, our results support Fraxinellone as a novel drug candidate in the treatment of colonic inflammation.
Vasorelaxing effect in rat thoracic aorta caused by fraxinellone and dictamine isolated from the Chinese herb Dictamnus dasycarpus Turcz: comparison with cromakalim and Ca2+ channel blockers.[Pubmed:1377790]
Naunyn Schmiedebergs Arch Pharmacol. 1992 Mar;345(3):349-55.
The components of Dictamnus dasycarpus Turcz were tested for their vasorelaxing effect on the rat aorta, and Fraxinellone and dictamine were shown to be effective vasorelaxants. In high K+ (60 mmol/l) medium, Ca2+ (0.03 to 3 mmol/l)-induced vasoconstriction was inhibited concentration-dependently by both agents. The IC50 for Fraxinellone and dictamine were calculated to be about 25 mumol/l and 15 mumol/l (for Ca2+ concentration of 1 mmol/l), respectively. Cromakalim (0.2-10 mumol/l) relaxed aortic rings precontracted with 15 but not 60 mmol/l of K+. Fraxinellone and verapamil were more potent and effective in producing relaxation in 60 mmol/l than in 15 mmol/l K(+)-induced contraction. However, dictamine was more potent in producing relaxation in 15 mmol/l K(+)-induced contraction. Nifedipine (1 mumol/l), dictamine (100 mumol/l) and Fraxinellone (100 mumol/l) relaxed the aortic contraction caused by KCl or Bay K 8644. The tonic contraction elicited by noradrenaline (NA, 3 mumol/l) was also relaxed by dictamine (500 mumol/l), but not by Fraxinellone (500 mumol/l) in the nifedipine (1 mumol/l)-treated aorta. This relaxing effect of dictamine persisted in endothelium-denuded aorta. Glibenclamide (10 mumol/l) shifted the concentration-relaxation curve of cromakalim, but not that of dictamine, to the right in rat aortic rings precontracted with NA. Dictamine (500 mumol/l) did not affect tonic contraction of NA which are reduced by H-7 (1 mumol/l) in Ca(2+)-depleted medium. In conclusion, Fraxinellone is a selective blocker of voltage-dependent Ca2+ channel, while dictamine relaxed the rat aorta by suppressing the Ca2+ influx through both voltage-dependent and receptor-operated Ca2+ channels.
Selective triggering of apoptosis of concanavalin A-activated T cells by fraxinellone for the treatment of T-cell-dependent hepatitis in mice.[Pubmed:19428326]
Biochem Pharmacol. 2009 Jun 1;77(11):1717-24.
Selectively inducing apoptosis of activated T cells is essential for the clearance of pathogenic injurious cells and subsequent efficient resolution of inflammation. However, few chemicals have been reported to trigger apoptosis of activated T cells in the treatment of hepatitis without affecting quiescent T cells. In the present study, we found that Fraxinellone, a small natural compound isolated from the root bark of Dictamnus dasycarpus, selectively facilitated apoptosis of concanavalin A (Con A)-activated CD4(+) T cells rather than those non-activated, by disrupting the mitochondrial transmembrane potential, decreasing the ratio of Bcl-2/Bax, and increasing cytochrome c release from the mitochondria to the cytosol. The enhancement in Fas expression and caspase-8 activity, truncation of Bid, and down-regulation of anti-apoptotic cellular FLICE-inhibitory protein expression by Fraxinellone also suggested the participation of an extrinsic apoptosis pathway. Furthermore, Fraxinellone significantly alleviated Con A-induced T-cell-dependent hepatitis in mice, which was closely associated with reduced serum transaminases, pro-inflammatory cytokines, and pathologic parameters. Consistent with the in vitro results, Fraxinellone dramatically induced apoptosis of activated peripheral CD4(+) T cells in vivo, consequently resulting in less CD4(+) T-cell activation and infiltration to the liver. These results strongly suggest Fraxinellone might be a potential leading compound useful in treating T-cell-mediated liver disorders in humans.


