OxcarbazepineBTX inhibitor CAS# 28721-07-5 |
- Daptomycin
Catalog No.:BCC1057
CAS No.:103060-53-3
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Clofarabine
Catalog No.:BCC1078
CAS No.:123318-82-1
- Ifosfamide
Catalog No.:BCC1164
CAS No.:3778-73-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 28721-07-5 | SDF | Download SDF |
| PubChem ID | 34312 | Appearance | Powder |
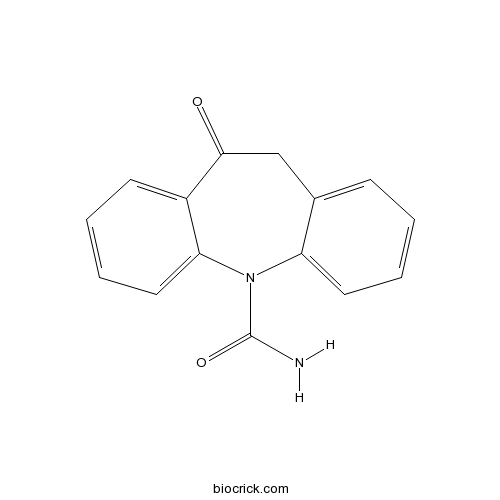
| Formula | C15H12N2O2 | M.Wt | 252.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 50 mg/mL (198.20 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
| Chemical Name | 5-oxo-6H-benzo[b][1]benzazepine-11-carboxamide | ||
| SMILES | C1C2=CC=CC=C2N(C3=CC=CC=C3C1=O)C(=O)N | ||
| Standard InChIKey | CTRLABGOLIVAIY-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H12N2O2/c16-15(19)17-12-7-3-1-5-10(12)9-14(18)11-6-2-4-8-13(11)17/h1-8H,9H2,(H2,16,19) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Anticonvulsant; protects mice and rats against generalized tonic-clonic seizures induced by electroshock. Thought to act via inhibition of sodium channel activity. |

Oxcarbazepine Dilution Calculator

Oxcarbazepine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.964 mL | 19.82 mL | 39.6401 mL | 79.2801 mL | 99.1002 mL |
| 5 mM | 0.7928 mL | 3.964 mL | 7.928 mL | 15.856 mL | 19.82 mL |
| 10 mM | 0.3964 mL | 1.982 mL | 3.964 mL | 7.928 mL | 9.91 mL |
| 50 mM | 0.0793 mL | 0.3964 mL | 0.7928 mL | 1.5856 mL | 1.982 mL |
| 100 mM | 0.0396 mL | 0.1982 mL | 0.3964 mL | 0.7928 mL | 0.991 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Oxcarbazepine inhibits the binding of [3H]BTX to sodium channels with IC50 of 160 μM and also inhibits the influx of 22Na+ into rat brain synaptosomes with IC50 about 100 μM.
- MA 2029
Catalog No.:BCC7983
CAS No.:287206-61-5
- PD 180970
Catalog No.:BCC3894
CAS No.:287204-45-9
- NCX 4040
Catalog No.:BCC7944
CAS No.:287118-97-2
- Ezatiostat hydrochloride
Catalog No.:BCC4259
CAS No.:286942-97-0
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
- 6'-Amino-3',4'-(methylenedioxy)acetophenone
Catalog No.:BCC8760
CAS No.:28657-75-2
- Epoxylathyrol
Catalog No.:BCN5382
CAS No.:28649-60-7
- Euphorbiasteroid
Catalog No.:BCN2781
CAS No.:28649-59-4
- Multicaulisin
Catalog No.:BCN7840
CAS No.:286461-76-5
- L-838,417
Catalog No.:BCC7617
CAS No.:286456-42-6
- Meloscandonine
Catalog No.:BCN5186
CAS No.:28645-27-4
- Nigericin sodium salt
Catalog No.:BCC7915
CAS No.:28643-80-3
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- 3CAI
Catalog No.:BCC5402
CAS No.:28755-03-5
- Apigenin 5-O-beta-D-glucopyranoside
Catalog No.:BCN5185
CAS No.:28757-27-9
- Rosuvastatin
Catalog No.:BCC4139
CAS No.:287714-41-4
- 4,5,6,7-Tetrahydrothieno [3,2,c]pyridine hydrochloride
Catalog No.:BCC8664
CAS No.:28783-41-7
- Nordihydrocapsaicin
Catalog No.:BCN2387
CAS No.:28789-35-7
- H-Cys(Acm)-OH.HCl
Catalog No.:BCC2903
CAS No.:28798-28-9
- Peptone, bacteriological
Catalog No.:BCC1210
CAS No.:288-88-0
- Tetrazole
Catalog No.:BCC2847
CAS No.:288-94-8
- Heraclenin
Catalog No.:BCN5187
CAS No.:2880-49-1
- 4,7-Bis(5-bromo-2-thienyl)-2,1,3-benzothiadiazole
Catalog No.:BCC8668
CAS No.:288071-87-4
- Fraxinellone
Catalog No.:BCN1272
CAS No.:28808-62-0
Transition from oxcarbazepine to eslicarbazepine acetate: A single center study.[Pubmed:28293474]
Brain Behav. 2017 Jan 27;7(3):e00634.
OBJECTIVES: There is limited clinical evidence for comparison between Oxcarbazepine (OXC) and eslicarbazepine acetate (ESL) in terms of tolerability, or how to execute the change from OXC to ESL. We report the process of transitioning patients with focal epilepsy from previous OXC treatment to ESL due to tolerability problems. The rationale for change from OXC is reported, and the outcome with respective to this rationale is analyzed in terms of tolerability and efficacy. MATERIALS AND METHODS: The subjects were transitioned overnight from OXC to ESL in a hospital inpatient setting. An evaluation of the effects of the transition was made after 1 and 3 months. All adverse events (AEs) were recorded following the transition period. Subjects were classified by outcome in terms of AEs. RESULTS: Twenty-three subjects were transitioned from OXC to ESL. Fifteen patients OXC-related AEs reduced significantly after transition. Particularly, most of (93%) the AEs presented in the morning resolved after transition to ESL. No patient had an increase in seizure frequency following the transition. The incidence of ESL-related AEs was 39% at 1 month and 13% at 3 month follow-up; however, all patients continued ESL throughout the study period. CONCLUSIONS: This study demonstrates that patients suffering from OXC-related AEs improve in terms of tolerability after a switch to ESL with maintaining seizure control. This improvement is more pronounced if the OXC-related AEs are most evident following morning dosing of OXC. Transition can be safely executed in an outpatient setting.
Divergence Palsy due to Divalproex and Oxcarbazepine.[Pubmed:28277442]
Clin Neuropharmacol. 2017 May/Jun;40(3):154-155.
OBJECTIVE: This case series is the first to describe divergence palsy as an adverse effect of antiepileptic drug use. Diplopia is a common adverse effect of antiepileptic drugs, but no explanatory motility deficit has ever been reported. METHODS: We present 2 patients, 1 on Oxcarbazepine and 1 on divalproex, each with a normal examination result between spells and divergency palsy when symptomatic. RESULTS: Discontinuation of the antiepileptic medication led to resolution of the episodes in both cases. Rechallenge with the offending agent after washout in one patient resulted in recurrence of diplopia and divergence palsy, both resolving after subsequent withdrawal of the antiepileptic. CONCLUSIONS: Antiepileptic drugs may cause divergence palsy.
Efficacy and safety of oxcarbazepine in the treatment of children with epilepsy: a meta-analysis of randomized controlled trials.[Pubmed:28293110]
Neuropsychiatr Dis Treat. 2017 Mar 2;13:685-695.
BACKGROUND: To assess the efficacy and safety of Oxcarbazepine (OXC) in the treatment of children with epilepsy. METHODS: Randomized controlled trials (RCTs) published in PubMed, Embase, Web of Science, Cochrane Library, Scopus, SinoMed (Chinese BioMedical Literature Service System, China), and Chinese National Knowledge Infrastructure (China) database were systematically reviewed. Eligible studies were those that compared the efficacy and safety of OXC with other antiepileptic drugs in epilepsy. Risk ratio (RR) with 95% confidence intervals (95% CIs) was calculated using fixed-effects or random-effects model. RESULTS: Eleven RCTs with a total of 1,241 patients met the inclusion criteria and were included in this meta-analysis. Compared with other antiepileptic drugs (sodium valproate, levetiracetam, phenytoin, and placebo), OXC was associated with similar seizure-free rate (RR =1.06, 95% CI: 0.94, 1.20; P=0.366) and percentage reduction from baseline in seizure frequency (for >/=75% reduction: RR =1.15, 95% CI: 0.88, 1.49; P=0.310; for 50%-75% reduction: RR =1.12, 95% CI: 0.90, 1.39; P=0.301; for <50% reduction: RR =0.79, 95% CI: 0.56, 1.12; P=0.179). Moreover, patients treated with OXC had a comparable incidence of adverse events compared with those treated with other antiepileptic drugs (RR =1.01, 95% CI: 0.92, 1.11; P=0.760). CONCLUSION: OXC showed similar effects and safety as other antiepileptic drugs in the treatment of children with epilepsy. Further well-conducted, large-scale RCTs are needed to validate these findings.
Oxcarbazepine, not its active metabolite, potentiates GABAA activation and aggravates absence seizures.[Pubmed:18717705]
Epilepsia. 2009 Jan;50(1):83-7.
PURPOSE: Studies in genetic absence epileptic rats from Strasbourg (GAERS) indicate that enhancement of gamma aminobutyric acid (GABA(A)) receptor activity is a critical mechanism in the aggravation of seizures by carbamazepine (CBZ). We examined whether structural analogs of CBZ, Oxcarbazepine (OXC), and its active metabolite, monohydroxy derivative (MHD), also potentiate GABA(A) receptor current and aggravate seizures. METHODS: In vitro studies in Xenopus oocytes compared the three drugs' effect on GABA(A) receptor currents. In vivo studies compared seizure activity in GAERS after intraperitoneal drug administration. RESULTS: OXC potentiated GABA(A) receptor current and aggravated seizures in GAERS, similarly to the effect of CBZ. Conversely, MHD showed only a minor potentiation of GABA(A) receptor current and did not aggravate seizures. DISCUSSION: A hydroxyl group at the C-10 position on the CBZ tricyclic structure in MHD reduces GABA(A) receptor potentiation and seizure aggravation. Reports of the aggravation of absence seizures in patients taking OXC may result from circulating unmetabolized OXC rather than MHD.
Mechanisms of action of carbamazepine and its derivatives, oxcarbazepine, BIA 2-093, and BIA 2-024.[Pubmed:11926264]
Neurochem Res. 2002 Feb;27(1-2):121-30.
Carbamazepine (CBZ) has been extensively used in the treatment of epilepsy, as well as in the treatment of neuropathic pain and affective disorders. However, the mechanisms of action of this drug are not completely elucidated and are still a matter of debate. Since CBZ is not very effective in some epileptic patients and may cause several adverse effects, several antiepileptic drugs have been developed by structural variation of CBZ, such as Oxcarbazepine (OXC), which is used in the treatment of epilepsy since 1990. (S)-(-)-10-acetoxy-10,11-dihydro-5H-dibenz [b,f]azepine-5-carboxamide (BIA 2-093) and 10,11-dihydro-10-hydroxyimino-5H-dibenz[b,f] azepine-5-carboxamide (BIA 2-024), which were recently developed by BIAL, are new putative antiepileptic drugs, with some improved properties. In this review, we will focus on the mechanisms of action of CBZ and its derivatives, OXC, BIA 2-093 and BIA 2-024. The available data indicate that the anticonvulsant efficacy of these AEDs is mainly due to the inhibition of sodium channel activity.
Oxcarbazepine: preclinical anticonvulsant profile and putative mechanisms of action.[Pubmed:8039471]
Epilepsia. 1994;35 Suppl 5:S47-50.
Oxcarbazepine (OCBZ, Trileptal) and its main human monohydroxy metabolite (MHD) protected mice and rats against generalized tonic-clonic seizures induced by electroshock with ED50 values between 13.5 and 20.5 mg/kg p.o. No tolerance toward this anticonvulsant effect was observed when rats were treated with OCBZ or MHD daily for 4 weeks. The therapeutic indices were 4 (OCBZ) and > 6 (MHD) for sedation (observation test, mice and rats) and 8 (MHD) or 10 (OCBZ) for motor impairment (rotorod test, mice). Both compounds were less potent in suppressing chemically induced seizures and did not significantly influence rat kindling development. At doses of 50 mg/kg p.o. and 20 mg/kg i.m. and higher, OCBZ and, to a lesser extent, MHD protected Rhesus monkeys from aluminum-induced chronically recurring partial seizures. In vitro, OCBZ and MHD suppressed sustained high-frequency repetitive firing of sodium-dependent action potentials in mouse neurons in cell culture with equal potency (medium effective concentration 5 x 10(-8) M/L). This effect is probably due in part to a direct effect on sodium channels. Patch-clamp studies on rat dorsal root ganglia cells revealed that up to a concentration of 3 x 10(-4) M, MHD did not significantly interact with L-type calcium currents, whereas OCBZ diminished them by about 30% at the concentration of 3 x 10(-4) M. In biochemical investigations, no brain neurotransmitter or modulator receptor site responsible for the anticonvulsant mechanism of action of OCBZ and MHD was identified.(ABSTRACT TRUNCATED AT 250 WORDS)


