MA 2029Potent and selective, competitive motilin receptor antagonist; orally active CAS# 287206-61-5 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 287206-61-5 | SDF | Download SDF |
| PubChem ID | 10144175 | Appearance | Powder |
| Formula | C31H45FN4O4 | M.Wt | 556.71 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in 1eq. HCl and to 100 mM in DMSO | ||
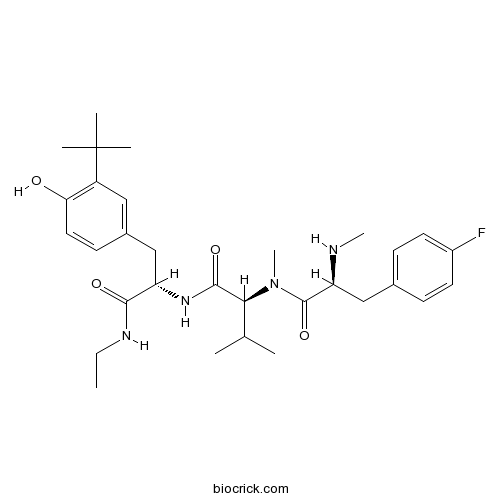
| Chemical Name | (2S)-N-[(2S)-3-(3-tert-butyl-4-hydroxyphenyl)-1-(ethylamino)-1-oxopropan-2-yl]-2-[[(2S)-3-(4-fluorophenyl)-2-(methylamino)propanoyl]-methylamino]-3-methylbutanamide | ||
| SMILES | CCNC(=O)C(CC1=CC(=C(C=C1)O)C(C)(C)C)NC(=O)C(C(C)C)N(C)C(=O)C(CC2=CC=C(C=C2)F)NC | ||
| Standard InChIKey | KSDWAJPUTRBIRR-KLJDGLGGSA-N | ||
| Standard InChI | InChI=1S/C31H45FN4O4/c1-9-34-28(38)24(18-21-12-15-26(37)23(16-21)31(4,5)6)35-29(39)27(19(2)3)36(8)30(40)25(33-7)17-20-10-13-22(32)14-11-20/h10-16,19,24-25,27,33,37H,9,17-18H2,1-8H3,(H,34,38)(H,35,39)/t24-,25-,27-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective, competitive motilin receptor antagonist (IC50 = 4.9 nM). Selective for the motilin receptor over a range of other receptors and ion channels. Inhibits motilin-induced duodenal muscle contractions in vitro. Also inhibits motilin-induced colonic and abdominal contractions in vivo. Orally active. |

MA 2029 Dilution Calculator

MA 2029 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7963 mL | 8.9813 mL | 17.9627 mL | 35.9253 mL | 44.9067 mL |
| 5 mM | 0.3593 mL | 1.7963 mL | 3.5925 mL | 7.1851 mL | 8.9813 mL |
| 10 mM | 0.1796 mL | 0.8981 mL | 1.7963 mL | 3.5925 mL | 4.4907 mL |
| 50 mM | 0.0359 mL | 0.1796 mL | 0.3593 mL | 0.7185 mL | 0.8981 mL |
| 100 mM | 0.018 mL | 0.0898 mL | 0.1796 mL | 0.3593 mL | 0.4491 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- PD 180970
Catalog No.:BCC3894
CAS No.:287204-45-9
- NCX 4040
Catalog No.:BCC7944
CAS No.:287118-97-2
- Ezatiostat hydrochloride
Catalog No.:BCC4259
CAS No.:286942-97-0
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
- 6'-Amino-3',4'-(methylenedioxy)acetophenone
Catalog No.:BCC8760
CAS No.:28657-75-2
- Epoxylathyrol
Catalog No.:BCN5382
CAS No.:28649-60-7
- Euphorbiasteroid
Catalog No.:BCN2781
CAS No.:28649-59-4
- Multicaulisin
Catalog No.:BCN7840
CAS No.:286461-76-5
- L-838,417
Catalog No.:BCC7617
CAS No.:286456-42-6
- Meloscandonine
Catalog No.:BCN5186
CAS No.:28645-27-4
- Nigericin sodium salt
Catalog No.:BCC7915
CAS No.:28643-80-3
- KRN 633
Catalog No.:BCC2544
CAS No.:286370-15-8
- Oxcarbazepine
Catalog No.:BCC5077
CAS No.:28721-07-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- 3CAI
Catalog No.:BCC5402
CAS No.:28755-03-5
- Apigenin 5-O-beta-D-glucopyranoside
Catalog No.:BCN5185
CAS No.:28757-27-9
- Rosuvastatin
Catalog No.:BCC4139
CAS No.:287714-41-4
- 4,5,6,7-Tetrahydrothieno [3,2,c]pyridine hydrochloride
Catalog No.:BCC8664
CAS No.:28783-41-7
- Nordihydrocapsaicin
Catalog No.:BCN2387
CAS No.:28789-35-7
- H-Cys(Acm)-OH.HCl
Catalog No.:BCC2903
CAS No.:28798-28-9
- Peptone, bacteriological
Catalog No.:BCC1210
CAS No.:288-88-0
- Tetrazole
Catalog No.:BCC2847
CAS No.:288-94-8
- Heraclenin
Catalog No.:BCN5187
CAS No.:2880-49-1
- 4,7-Bis(5-bromo-2-thienyl)-2,1,3-benzothiadiazole
Catalog No.:BCC8668
CAS No.:288071-87-4
Cardiovascular safety profile of MA-2029, a novel motilin receptor antagonist.[Pubmed:19043284]
J Toxicol Sci. 2008 Dec;33(5):631-9.
The aim of this study was to assess the cardiovascular effect of MA-2029, a selective motilin receptor antagonist highly expected for the treatment of irritable bowel syndrome (IBS). MA-2029 inhibited the human ether-a-go-go-related gene (hERG) current at 100 microg/ml, but shortened action potential duration (APD) in isolated guinea pig papillary muscles at 10 and 100 microg/ml and the corrected QT (QTc) interval after oral administration of 30 and 300 mg/kg in conscious telemetered dogs. The discrepancy was probably caused by blockade of the Ca(2+) channel because MA-2029 inhibited the Ca(2+) current in isolated guinea pig myocytes. MA-2029 at 100 microg/ml also decreased the maximum rising velocity and action potential amplitude in the action potential study, indicating that MA-2029 has Na(+) channel blocking potential. In the cardiovascular study, MA-2029 at 30 mg/kg induced slight cardiovascular changes such as hypotension, QTc shortening, and PR prolongation possibly caused by Ca(2+) channel blockade. The plasma concentration at 4 hr after 30 mg/kg administration was 2.10 microg/ml, 200-fold higher than the effective concentration of MA-2029 as a motilin receptor antagonist. These results suggest that MA-2029 has sufficient cardiovascular safety although it inhibits multiple ion channels at supra-effective concentrations. On the other hand, cisapride, an effective IBS drug, showed clear hERG inhibition and APD prolongation at 100 ng/ml. Cisapride exhibited a narrow safety margin because it caused QT prolongation potential even at the therapeutic concentration. In conclusion, MA-2029 is a novel drug highly expected for the treatment of IBS with lower cardiovascular risk than cisapride.
Oral administration of MA-2029, a novel selective and competitive motilin receptor antagonist, inhibits motilin-induced intestinal contractions and visceral pain in rabbits.[Pubmed:18164286]
Eur J Pharmacol. 2008 Mar 10;581(3):296-305.
The pharmacological properties of MA-2029, a novel motilin receptor antagonist, were investigated. In vitro, MA-2029 (1 to 30 nM) competitively inhibited motilin-induced contractions in isolated rabbit duodenal longitudinal muscle strips, with a pA2 value of 9.17+/-0.01 (n=5). However, contractile responses to acetylcholine and substance P were unaffected even at 1 microM of MA-2029. MA-2029 concentration-dependently inhibited the binding of [125 I]motilin to motilin receptors in a homogenate of rabbit colon smooth muscle tissue and membranes of HEK 293 cells expressing human motilin receptors. The pKi of MA-2029 was 8.58+/-0.04 in the rabbit colon homogenate (n=4) and 8.39 in the HEK 293 cells (mean of duplicate experiments). In vivo, orally-administered MA-2029 (3 to 30 mg/kg) dose-dependently inhibited colonic contractions induced by motilin (3 microg/kg, i.v.) in conscious rabbits. Inhibition was caused by all doses at 30 min after administration and by 10 mg/kg or more at 4 h after administration. The plasma concentration of MA-2029 correlated with its inhibitory effect. Furthermore, the oral administration of MA-2029 (0.3 to 3 mg/kg) also inhibited abdominal muscle contractions (an index of the visceral pain) induced by intravenous infusion of motilin (3 microg/kg/h) during colorectal distension in conscious rabbits. These results indicate that MA-2029 is an orally active, selective and competitive motilin receptor antagonist. It is suggested that this compound may be useful for gastrointestinal disorders associated with disturbed gastrointestinal motility such as irritable bowel syndrome.
An orally active motilin receptor antagonist, MA-2029, inhibits motilin-induced gastrointestinal motility, increase in fundic tone, and diarrhea in conscious dogs without affecting gastric emptying.[Pubmed:19445919]
Eur J Pharmacol. 2009 Aug 1;615(1-3):185-92.
The pharmacological properties of MA-2029, a selective and competitive motilin receptor antagonist, were investigated in conscious dogs after oral administration. Gastrointestinal contractile activity was recorded by chronically implanted force transducers. The proximal gastric volume was measured with a barostat under constant pressure. Gastric emptying was examined using the paracetamol absorption test. MA-2029 (0.3-10 mg/kg, p.o.) administered in the interdigestive state inhibited gastrointestinal contractions induced by motilin (3 microg/kg, i.v.) in a dose-dependent manner. MA-2029 (0.3-3 mg/kg, p.o.) also inhibited the occurrence of spontaneous phase III contractions, even though MA-2029 had no effect on basal gastrointestinal motility or basal gastric emptying even at 10 and 30 mg/kg p.o. The inhibitory effect of MA-2029 on motilin-induced gastrointestinal motility corresponded to its plasma concentration. Motilin (0.3 microg/kg/h, i.v. infusion) reduced the proximal gastric volume by about 50% of control during isobaric distension. This effect was also inhibited by MA-2029 (1-10 mg/kg, p.o.) in a dose-dependent manner. In the digestive state, injection of motilin (3 microg/kg, i.v.) induced diarrhea in 9 of 11 dogs. MA-2029 (1-30 mg/kg, p.o.) reduced the incidence of diarrhea induced by motilin in a dose-dependent manner. The results indicate that MA-2029 inhibits hypermotility induced by motilin in conscious dogs without having an effect on the basal gastrointestinal tone or gastric emptying rate. MA-2029 may be useful in treating gastrointestinal disorders in which the pathogenesis involves the elevation of circulating motilin.


