Pracinostat (SB939)Pan-HDAC inhibitor CAS# 929016-96-6 |
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 929016-96-6 | SDF | Download SDF |
| PubChem ID | 49855250 | Appearance | Powder |
| Formula | C20H30N4O2 | M.Wt | 358.48 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | SB939 | ||
| Solubility | DMSO : 50 mg/mL (139.48 mM; Need ultrasonic) | ||
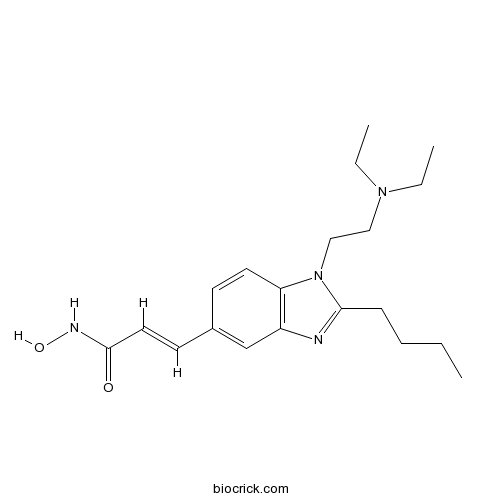
| Chemical Name | (E)-3-[2-butyl-1-[2-(diethylamino)ethyl]benzimidazol-5-yl]-N-hydroxyprop-2-enamide | ||
| SMILES | CCCCC1=NC2=C(N1CCN(CC)CC)C=CC(=C2)C=CC(=O)NO | ||
| Standard InChIKey | JHDKZFFAIZKUCU-ZRDIBKRKSA-N | ||
| Standard InChI | InChI=1S/C20H30N4O2/c1-4-7-8-19-21-17-15-16(10-12-20(25)22-26)9-11-18(17)24(19)14-13-23(5-2)6-3/h9-12,15,26H,4-8,13-14H2,1-3H3,(H,22,25)/b12-10+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pracinostat (SB939) is a potent inhibitor of HDAC with IC50 of 40-140 nM with exception for HDAC6. | |||||
| Targets | HDAC1 | HDAC3 | HDAC4 | HDAC5 | HDAC9 | HDAC10 |
| IC50 | 49 nM | 43 nM | 56 nM | 47 nM | 70 nM | 40 nM |
| Cell experiment: | |
| Cell lines | Ovarian (A2780) , colon (HCT-116), and prostate (PC-3) cell lines |
| Preparation method | The solubility of this compound in DMSO is >10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37 °C for 10 minutes and/or shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. |
| Reacting condition | 96 h; IC50=0.48±0.21 μM (A2780), 0.48±0.27 μM (HCT-116), 0.34±0.06 μM (PC-3) |
| Applications | SB939 showed broad anti-proliferative activity against representative tumor cells from ovarian (A2780) , colon (HCT-116), and prostate (PC-3) with cellular IC50 values of 0.48±0.21, 0.48±0.27 and 0.34±0.06 μM, respectively. |
| Animal experiment: | |
| Animal models | Athymic nude mice |
| Dosage form | 200 mg/kg, 100 mg/kg, 50 mg/kg; oral taken. |
| Application | SB939 was clearly toxic at the highest dose tested (200 mg/kg); however, at the MTD dose of 100 mg/kg and at 50mg/kg, it demonstrated very significant antitumor effects on day 21 with TGI = 90% (p < 0.001 ) and 66% ( p < 0.001), respectively, with acceptable body weight loss. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: [1] Wang H, Yu N, Chen D, et al. Discovery of (2 E)-3-{2-butyl-1-[2-(diethylamino) ethyl]-1 H-benzimidazol-5-yl}-N-hydroxyacrylamide (SB939), an orally active histone deacetylase inhibitor with a superior preclinical profile[J]. Journal of medicinal chemistry, 2011, 54(13): 4694-4720. | |

Pracinostat (SB939) Dilution Calculator

Pracinostat (SB939) Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7896 mL | 13.9478 mL | 27.8956 mL | 55.7911 mL | 69.7389 mL |
| 5 mM | 0.5579 mL | 2.7896 mL | 5.5791 mL | 11.1582 mL | 13.9478 mL |
| 10 mM | 0.279 mL | 1.3948 mL | 2.7896 mL | 5.5791 mL | 6.9739 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.5579 mL | 1.1158 mL | 1.3948 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.5579 mL | 0.6974 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
SB939 is a HDAC that potently inhibited the growth of multiple malarial parasites at different life stages.
Abstract
The preclinical ADME properties of SB939, a orally active HDAC inhibitor primarily metabolized by CYP3A4 and CYP1A2 in human, have been characterized in human and animals, where observed ADME of SB939 in human were consistent with predication s by Simcyp and allometric scaling.
Abstract
SB939, a HDAC, has been assessed for safety, MTD, PKs, PDs and efficacy in pateients with advanced solid malignancies.
Abstract
With a RP2D of 60 mg po t.i.w. for 3 weeks every 4 weeks in adult patients, the toxicities, PKs and RP2D of SB939, an inhibitor of class 1/2/4 HDAC, have been investigated in children with refractory solid tumors.
Abstract
SB939, an inhibitor of class 1/2/4 HDACs, exhibited favorable PKs and PDs in preclinical models leading to successful applications to patients, where SB939 dose- and time-dependently induced acH3 accumulation in cancerous tissues and leukemic bone marrow.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Pracinostat, also known as SB939, is a potent and orally available inhibitor of histone deacetylase (HDAC) with a relatively stronger selectivity (more than 1000-fold) for class I, class II and class IV HDACs rather than class III HDACs. Pracinostat potently suppresses proliferation in a wide range of cancer cell lines, including colon cancer, ovarian cancer, prostate carcinomas, acute myeloid leukaemia (AML) and B cell lymphoma. Recent study results have shown that SB939 induces the accumulation of acetylated histone H3 (AcH3) and acetylated α-tubulin and increases the expression of the cyclin dependent kinase inhibitor p21 in cancer cells.
Reference
Razak AR, Hotte SJ, Siu LL, Chen EX, Hirte HW, Powers J, Walsh W, Stayner LA, Laughlin A, Novotny-Diermayr V, Zhu J, Eisenhauer EA. Phase I clinical, pharmacokinetic and pharmacodynamic study of SB939, an oral histone deacetylase (HDAC) inhibitor, in patients with advanced solid tumours. Br J Cancer. 2011;104(5):756-762.
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
- PF-03716556
Catalog No.:BCC2084
CAS No.:928774-43-0
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Tenacissoside F
Catalog No.:BCN4472
CAS No.:928151-78-4
- Alisol O
Catalog No.:BCN3362
CAS No.:928148-51-0
- Golvatinib (E7050)
Catalog No.:BCC4423
CAS No.:928037-13-2
- RAF265
Catalog No.:BCC3677
CAS No.:927880-90-8
- DPNI-caged-GABA
Catalog No.:BCC5957
CAS No.:927866-58-8
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
- 7-Oxo-ganoderic acid Z
Catalog No.:BCN7973
CAS No.:929248-72-6
- LUF 6283
Catalog No.:BCC6318
CAS No.:92933-48-7
- Fmoc-Tyr-OH
Catalog No.:BCC3562
CAS No.:92954-90-0
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
- Sessilifoline A
Catalog No.:BCN4473
CAS No.:929637-35-4
- Cucumegastigmane I
Catalog No.:BCN4474
CAS No.:929881-46-9
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
- 3,4-Dimethoxybenzoic acid
Catalog No.:BCN4475
CAS No.:93-07-2
- 2-Acetonaphthone
Catalog No.:BCC8510
CAS No.:93-08-3
A phase I study of histone deacetylase inhibitor, pracinostat (SB939), in pediatric patients with refractory solid tumors: IND203 a trial of the NCIC IND program/C17 pediatric phase I consortium.[Pubmed:23893953]
Pediatr Blood Cancer. 2013 Nov;60(11):1868-74.
BACKGROUND: Pracinostat (SB939) is a potent oral inhibitor of class 1, 2, and 4 histone deacetylases (HDAC). The adult recommended phase II dose (RP2D) is 60 mg po three times per week (t.i.w.) for 3 weeks every 4 weeks. This study assessed the toxicities and pharmacokinetics of pracinostat and determined the RP2D in children with refractory solid tumors. METHODS: Pediatric patients with refractory solid tumors were treated with oral pracinostat t.i.w. for 3 consecutive weeks, followed by 1 week off dosing. Three dose levels-25, 35, and 45 mg/m(2) were evaluated using a standard 3 + 3 cohort design. Pharmacokinetic (PK) studies were optional. RESULTS: Twelve patients were enrolled. The most common diagnosis was Ewing sarcoma. Most adverse events (AEs) were hematological with five (40%) patients experiencing grade 3 neutropenia. Non-hematological AEs were generally grade 1. No dose limiting toxicities occurred. More hematological and non-hematological AEs occurred at 45 mg/m(2) : Two of five patients experienced Grade 3 neutropenia and one each Grade 3 thrombocytopenia and leucopenia, Grade 1 fatigue and anorexia occurred in three. The RP2D was declared to be 45 mg/m(2) (comparable to an adult dose of 80 mg). One patient had a best response of stable disease (duration of 2.9 months). Three patients on 25 mg/m(2) and one each on 35 and 45 mg/m(2) participated in the PK study. No dose related changes in Cmax or AUC occurred. CONCLUSIONS: Pracinostat is reasonably well tolerated in children with refractory solid tumors. The RP2D is 45 mg/m(2) .
Preclinical metabolism and disposition of SB939 (Pracinostat), an orally active histone deacetylase inhibitor, and prediction of human pharmacokinetics.[Pubmed:21873472]
Drug Metab Dispos. 2011 Dec;39(12):2219-32.
The preclinical absorption, distribution, metabolism, and excretion (ADME) properties of Pracinostat [(2E)-3-[2-butyl-1-[2-(diethylamino) ethyl]-1H-benzimidazol-5-yl]-N-hydroxyarylamide hydrochloride; SB939], an orally active histone deacetylase inhibitor, were characterized and its human pharmacokinetics (PK) was predicted using Simcyp and allometric scaling. SB939 showed high aqueous solubility with high Caco-2 permeability. Metabolic stability was relatively higher in dog and human liver microsomes than in mouse and rat. The major metabolites formed in human liver microsomes were also observed in preclinical species. Human cytochrome P450 (P450) phenotyping showed that SB939 was primarily metabolized by CYP3A4 and CYP1A2. SB939 did not significantly inhibit human CYP3A4, 1A2, 2D6, and 2C9 (>25 muM) but inhibited 2C19 (IC(50) = 5.8 muM). No significant induction of human CYP3A4 and 1A2 was observed in hepatocytes. Plasma protein binding in mouse, rat, dog, and human ranged between approximately 84 and 94%. The blood-to-plasma ratio was approximately 1.0 in human blood. SB939 showed high systemic clearance (relative to liver blood flow) of 9.2, 4.5, and 1.5 l . h(-1) . kg(-1) and high volume of distribution at steady state (>0.6 l/kg) of 3.5, 1.7, and 4.2 l/kg in mouse, rat, and dog, respectively. The oral bioavailability was 34, 65, and approximately 3% in mice, dogs, and rats, respectively. The predicted oral PK profile and parameters of SB939, using Simcyp and allometric scaling, were in good agreement with observed data in humans. Simcyp predictions showed lack of CYP3A4 and 2C19 drug-drug interaction potential for SB939. In summary, the preclinical ADME of SB939 supported its preclinical and clinical development as an oral drug candidate.
The oral HDAC inhibitor pracinostat (SB939) is efficacious and synergistic with the JAK2 inhibitor pacritinib (SB1518) in preclinical models of AML.[Pubmed:22829971]
Blood Cancer J. 2012 May;2(5):e69.
Acute myeloid leukemia (AML) is currently treated with aggressive chemotherapy that is not well tolerated in many elderly patients, hence the unmet medical need for effective therapies with less toxicity and better tolerability. Inhibitors of FMS-like tyrosine kinase 3 (FLT3), JAK2 and histone deacetylase inhibitors (HDACi) have been tested in clinical studies, but showed only moderate single-agent activity. High efficacy of the HDACi pracinostat treating AML and synergy with the JAK2/FLT3 inhibitor pacritinib is demonstrated. Both compounds inhibit JAK-signal transducer and activator of transcription (STAT) signaling in AML cells with JAK2(V617F) mutations, but also diminish FLT3 signaling, particularly in FLT3-ITD (internal tandem duplication) cell lines. In vitro, this combination led to decreased cell proliferation and increased apoptosis. The synergy translated in vivo in two different AML models, the SET-2 megakaryoblastic AML mouse model carrying a JAK2(V617F) mutation, and the MOLM-13 model of FLT3-ITD-driven AML. Pracinostat and pacritinib in combination showed synergy on tumor growth, reduction of metastases and synergistically decreased JAK2 or FLT signaling, depending on the cellular context. In addition, several plasma cytokines/growth factors/chemokines triggered by the tumor growth were normalized, providing a rationale for combination therapy with an HDACi and a JAK2/FLT3 inhibitor for the treatment of AML patients, particularly those with FLT3 or JAK2 mutations.


