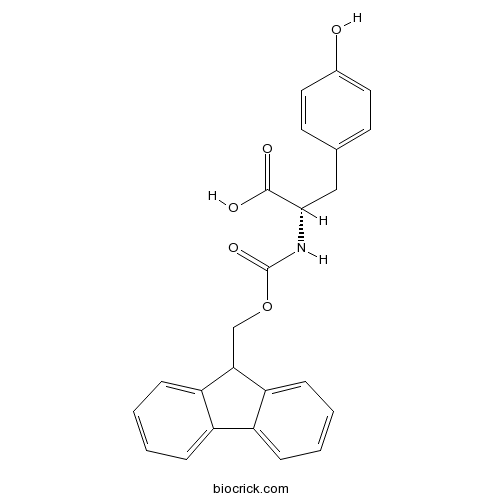
Fmoc-Tyr-OHCAS# 92954-90-0 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 92954-90-0 | SDF | Download SDF |
| PubChem ID | 6957986 | Appearance | Powder |
| Formula | C24H21NO5 | M.Wt | 403.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (2S)-2-(9H-fluoren-9-ylmethoxycarbonylamino)-3-(4-hydroxyphenyl)propanoic acid | ||
| SMILES | C1=CC=C2C(=C1)C(C3=CC=CC=C32)COC(=O)NC(CC4=CC=C(C=C4)O)C(=O)O | ||
| Standard InChIKey | SWZCTMTWRHEBIN-QFIPXVFZSA-N | ||
| Standard InChI | InChI=1S/C24H21NO5/c26-16-11-9-15(10-12-16)13-22(23(27)28)25-24(29)30-14-21-19-7-3-1-5-17(19)18-6-2-4-8-20(18)21/h1-12,21-22,26H,13-14H2,(H,25,29)(H,27,28)/t22-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Tyr-OH Dilution Calculator

Fmoc-Tyr-OH Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4789 mL | 12.3946 mL | 24.7893 mL | 49.5786 mL | 61.9732 mL |
| 5 mM | 0.4958 mL | 2.4789 mL | 4.9579 mL | 9.9157 mL | 12.3946 mL |
| 10 mM | 0.2479 mL | 1.2395 mL | 2.4789 mL | 4.9579 mL | 6.1973 mL |
| 50 mM | 0.0496 mL | 0.2479 mL | 0.4958 mL | 0.9916 mL | 1.2395 mL |
| 100 mM | 0.0248 mL | 0.1239 mL | 0.2479 mL | 0.4958 mL | 0.6197 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Tyr-OH
- LUF 6283
Catalog No.:BCC6318
CAS No.:92933-48-7
- 7-Oxo-ganoderic acid Z
Catalog No.:BCN7973
CAS No.:929248-72-6
- GSK461364
Catalog No.:BCC3788
CAS No.:929095-18-1
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
- DB07268
Catalog No.:BCC1519
CAS No.:929007-72-7
- PF-03716556
Catalog No.:BCC2084
CAS No.:928774-43-0
- IRAK inhibitor 2
Catalog No.:BCC1655
CAS No.:928333-30-6
- AS 1892802
Catalog No.:BCC6335
CAS No.:928320-12-1
- MN 64
Catalog No.:BCC6489
CAS No.:92831-11-3
- Boc-D-Asp-OBzl
Catalog No.:BCC3370
CAS No.:92828-64-3
- 3-Chloro-1-(4-octylphenyl)-propanone
Catalog No.:BCN2249
CAS No.:928165-59-7
- Tenacissoside F
Catalog No.:BCN4472
CAS No.:928151-78-4
- Bavisant
Catalog No.:BCC1402
CAS No.:929622-08-2
- Bavisant dihydrochloride
Catalog No.:BCC1403
CAS No.:929622-09-3
- Sessilifoline A
Catalog No.:BCN4473
CAS No.:929637-35-4
- Cucumegastigmane I
Catalog No.:BCN4474
CAS No.:929881-46-9
- SGI-110
Catalog No.:BCC2221
CAS No.:929901-49-5
- 3,4-Dimethoxybenzyl Alcohol
Catalog No.:BCN2721
CAS No.:93-03-8
- 3,4-Dimethoxybenzoic acid
Catalog No.:BCN4475
CAS No.:93-07-2
- 2-Acetonaphthone
Catalog No.:BCC8510
CAS No.:93-08-3
- Guaifenesin
Catalog No.:BCN2977
CAS No.:93-14-1
- Methyleugenol
Catalog No.:BCN4074
CAS No.:93-15-2
- Methyl isoeugenol
Catalog No.:BCN8462
CAS No.:93-16-3
- N-(2-Methoxyphenyl)acetamide
Catalog No.:BCC9054
CAS No.:93-26-5
Fmoc/solid-phase synthesis of Tyr(P)-containing peptides through t-butyl phosphate protection.[Pubmed:1717394]
Int J Pept Protein Res. 1991 Jun;37(6):572-5.
The synthesis of Tyr(P)-containing peptides by the use of Fmoc-Tyr(PO3Me2)-OH in Fmoc/solid phase synthesis is complicated since, firstly, piperidine causes cleavage of the methyl group from the -Tyr(PO3Me2)-residue during peptide synthesis and, secondly, harsh conditions are needed for its final cleavage. A very simple method for the synthesis of Tyr(P)-containing peptides using t-butyl phosphate protection is described. The protected phosphotyrosine derivative, Fmoc-Tyr(PO3tBu2)-OH was prepared in high yield from Fmoc-Tyr-OH by a one-step procedure which employed di-t-butyl N,N-diethyl-phosphoramidite as the phosphorylation reagent. The use of this derivative in Fmoc/solid phase peptide synthesis is demonstrated by the preparation of the Tyr(P)-containing peptides, Ala-Glu-Tyr(P)-Ser-Ala and Ser-Ser-Ser-Tyr(P)-Tyr(P).
Chemical synthesis of O-thiophosphotyrosyl peptides.[Pubmed:8200732]
Int J Pept Protein Res. 1994 Feb;43(2):146-53.
The synthon for O-thiophosphotyrosine, Fmoc-Tyr[PS(OBzl)2]-OH (1c), was prepared in 63% yield from Fmoc-Tyr-OH by first transient protection as the tBuMe2Si-ester and phosphinylation with (BzlO)2PNiPr2/tetrazole followed by oxidation of P(III) to P(V) with S8 in CS2. Building block 1c was incorporated in the Fmoc solid-phase synthesis of two O-thiophosphotyrosine-containing peptides H-Thr-Glu-Pro-Gln-Tyr(PS)-Gln-Pro-Gly-Glu-OH (2) and H-Thr-Arg-Asp-Ile-Tyr(PS)-Glu-Thr-Asp-Phe-Phe-Arg-Lys-OH (3), corresponding to sequences of the p60src (523-531) protein and an insulin receptor (IR) (1142-1153) analogue, respectively. An alternative approach of synthesis, the global phosphorylation of a resin-bound peptide, also proved useful. Thus, the free tyrosyl side-chain containing-peptide IR (1142-1153) on support was phosphinylated with the above phosphoramidite reagent followed by oxidation with either S8/CS2 or tetraethylthiuram disulfide/CH3CN solutions. Deprotection and peptide-resin cleavage was performed with a TFA/thiophenol (H2O) mixture. Crude peptides 2 and 3 were stable to the acidolytic deprotection. Preparative RP(C18)HPLC was initially performed using 0.1% TFA(aq)/EtOH solvents. However, analyses of fractions resulting from the purification step indicated significant decomposition of thiophosphopeptide in solution. Stability measurements both as a function of time and pH, further confirmed this initial finding. Purifications performed at intermediate pH using a triethylammonium acetate (pH 7.5)/CH3CN solvent system overcame this problem.
Efficient solid phase synthesis of mixed Thr(P)-, Ser(P)- and Tyr(P)-containing phosphopeptides by "global" "phosphite-triester" phosphorylation.[Pubmed:1280250]
Int J Pept Protein Res. 1992 Aug;40(2):134-40.
The synthesis of the mixed Thr(P)/Tyr(P)-containing peptide, Ala-Thr(P)-Tyr(P)-Ser-Ala, was accomplished by "phosphite-triester" phosphorylation of the resin-bound Thr/Tyr-containing peptide using di-t-butyl N,N-diethylphosphoramidite as the phosphitylation reagent. The pentapeptide-resin was assembled by Fmoc/solid-phase peptide synthesis with the use of PyBOP as coupling reagent and the hydroxy-amino acids incorporated as side-chain free Fmoc-Tyr-OH and Fmoc-Thr-OH. "Global" bis-phosphorylation of the peptide-resin was accomplished by treatment with di-t-butyl N,N-diethylphosphoramidite/1H-tetrazole followed by m-chloroperoxybenzoic acid oxidation of the intermediate di-t-butylphosphite triester. Simultaneous peptide-resin cleavage and peptide deprotection was effected by treatment of the peptide-resin with 5% anisole/TFA and gave the Thr(P)/Tyr(P)-containing phosphopeptide in high yield and purity. In addition, the tyrosyl residue was found to be phosphitylated in preference to the threonyl residue since the phosphitylation of the pentapeptide-resin using only 1.1 equiv. of di-t-butyl N,N-diethylphosphoramidite gave Ala-Thr-Tyr(P)-Ser-Ala as the major product and both Ala-Thr(P)-Tyr(P)-Ser-Ala and Ala-Thr-Tyr-Ser-Ala as minor products.


