M344HDAC inhibitor,potent and cell-permeable CAS# 251456-60-7 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- ITF2357 (Givinostat)
Catalog No.:BCC2150
CAS No.:732302-99-7
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Pracinostat (SB939)
Catalog No.:BCC2152
CAS No.:929016-96-6
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 251456-60-7 | SDF | Download SDF |
| PubChem ID | 3994 | Appearance | Powder |
| Formula | C16H25N3O3 | M.Wt | 307.39 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | D 237; MS 344 | ||
| Solubility | DMSO : ≥ 100 mg/mL (325.32 mM) *"≥" means soluble, but saturation unknown. | ||
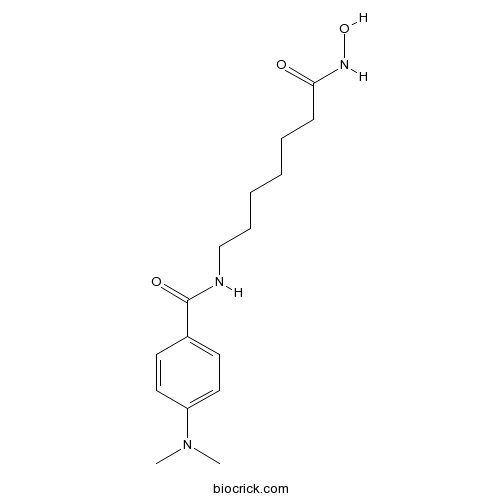
| Chemical Name | 4-(dimethylamino)-N-[7-(hydroxyamino)-7-oxoheptyl]benzamide | ||
| SMILES | CN(C)C1=CC=C(C=C1)C(=O)NCCCCCCC(=O)NO | ||
| Standard InChIKey | MXWDSZWTBOCWBK-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H25N3O3/c1-19(2)14-10-8-13(9-11-14)16(21)17-12-6-4-3-5-7-15(20)18-22/h8-11,22H,3-7,12H2,1-2H3,(H,17,21)(H,18,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Histone deacetylase inhibitor (IC50 = 100 nM). Induces terminal cell differentiation and causes an increase in hyperacetylated histone H4. Antiproliferative agent; suppresses the growth of human endometrial and ovarian cancer cells by inducing cell cycle arrest and apoptosis. |

M344 Dilution Calculator

M344 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.2532 mL | 16.266 mL | 32.532 mL | 65.0639 mL | 81.3299 mL |
| 5 mM | 0.6506 mL | 3.2532 mL | 6.5064 mL | 13.0128 mL | 16.266 mL |
| 10 mM | 0.3253 mL | 1.6266 mL | 3.2532 mL | 6.5064 mL | 8.133 mL |
| 50 mM | 0.0651 mL | 0.3253 mL | 0.6506 mL | 1.3013 mL | 1.6266 mL |
| 100 mM | 0.0325 mL | 0.1627 mL | 0.3253 mL | 0.6506 mL | 0.8133 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||
Abstract
The effects and mechanism of M344, a HDAC inhibitor with robust anticancer activity and low toxicity, on inducing HIV expression in latently infected cells are yet to be explored.
Abstract
M344 is an HDAC inhibitor that concentrate- and time-dependently decreases proliferation, reduces p53 mRNA expression without affecting p53 protein levels and induces expression of Puma and Bax with morphological features of apoptosis in MCF-7 breast cancer cells.
Abstract
The combination of vorinostat and temozolomide has been evaluated for MTD, DLT and pharmacokinetic properties in children with refractory or recurrent CNS malignancies.
Abstract
The combination of vorinostat and bortezomib has been assessed for efficacy and tolerability in patients with relapsed or refractory multiple myeloma.
Abstract
As an inhibitor of class I and II HDAC, vorinostat induces cell cycle arrest and apoptosis by altering expression of target genes.

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
M344 is a potent inhibitor of HDAC with IC50 value of 100 nM and enable the induction of cell differentiation [1].
Treatment with M344 for 1 or 3 days induced a decreased relative p53 mRNA level and increased p21waf1/cip1 mRNA expression while no change in p53 protein. The result demonstrated the independent of p53 of inhibitory effects of M344 on MCF-7 cell growth. And the increased expression of the pro-apoptotic Puma, which can be induced by p53-independent pathways, is in accordance with the suppression of MCF-7 cell growth observed after the M344 treatment. On the other hand, M344 also show the ability in increasing the response to radiation in SCC-35 and SQ-20B human squamous carcinoma lines [2].
In MEL DS19 cells, M344 shows a much more significant effect on cell proliferation than the effect on cell differentiation. M344 exhibits toxic at concentrations of above 10 μM, when only 20% of the surviving cell population at most are induced to differentiate. M344 significantly inhibits proliferation of embryonic nervous system tumor cells, including medulloblastoma cells (D341 MED) with GI50 value of 0.65 μM and neuroblastoma cells (CH-LA 90) with GI50 value of 0.63 μM [1, 3].
M344 also plays an important role in the modification of histone and transcription factor of NF- kB in regulating HIV-1 LTR gene expression, showing the potential anti-latency therapies. Experiments were carried out in the cells, which latently infected Jurkat cells encoding the green fluorescence protein (GFP) under control of the HIV-1 LTR that act as a marker of expression of HIV-1 LTR, 72 hours after treatment with 200 nM M344, expression of HIV-1 activity was found, and the percentage of GFP-expressing cells was detected as high as 25.2% more than the cells which was subjected to mock treatment [4].
References:
[1]. Jung M, Brosch G , Kolle D, et al. Amide analogues of trichostatin A as inhibitors of histone deacetylase and inducers of terminal cell differentiation. JOURNAL OF MEDICINAL CHEMISTRY, 1999, 42 (22): 4669-4679.
[2]. Yeung A, Bhargava RK, Ahn, R, et al. HDAC inhibitor M344 suppresses MCF-7 breast cancer cell proliferation. BIOMEDICINE & PHARMACOTHERAPY, 2012, 66 (3): 232-236.
[3]. Furchert SE, Lanvers-Kaminsky C , Jurgens H , et al. Inhibitors of histone deacetylases as potential therapeutic tools for high-risk embryonal tumors of the nervous system of childhood. INTERNATIONAL JOURNAL OF CANCER, 2007, 120 (8): 1787-1794.
[4]. Ying H, Zhang YH , Zhou X , et al. Selective Histonedeacetylase Inhibitor M344 Intervenes in HIV-1 Latency through Increasing Histone Acetylation and Activation of NF-kappaB. PLOS ONE, 2012, 7 (11): e48832.
- SU 16f
Catalog No.:BCC7639
CAS No.:251356-45-3
- Urotensin II (human)
Catalog No.:BCC5796
CAS No.:251293-28-4
- Crebanine
Catalog No.:BCN5117
CAS No.:25127-29-1
- FRATide
Catalog No.:BCC5821
CAS No.:251087-38-4
- Antazoline HCl
Catalog No.:BCC4627
CAS No.:2508-72-7
- Salvisyrianone
Catalog No.:BCN4821
CAS No.:250691-57-7
- NNC 63-0532
Catalog No.:BCC7177
CAS No.:250685-44-0
- Pedunsaponin A
Catalog No.:BCN8192
CAS No.:250613-27-5
- Cyclo(RGDyK)
Catalog No.:BCC6512
CAS No.:250612-42-1
- (±)-Acetylcarnitine chloride
Catalog No.:BCC6617
CAS No.:2504-11-2
- Excavatin M
Catalog No.:BCN5116
CAS No.:250293-31-3
- PNU 177864 hydrochloride
Catalog No.:BCC7664
CAS No.:250266-51-4
- Tesaglitazar
Catalog No.:BCC7828
CAS No.:251565-85-2
- 5-Chloro-2-nitrobenzoic acid
Catalog No.:BCC8743
CAS No.:2516-95-2
- Acevaltrate
Catalog No.:BCN7127
CAS No.:25161-41-5
- Loline
Catalog No.:BCN2003
CAS No.:25161-91-5
- Isohomoarbutin
Catalog No.:BCN7612
CAS No.:25162-30-5
- Dynamin inhibitory peptide
Catalog No.:BCC1034
CAS No.:251634-21-6
- AM 1172
Catalog No.:BCC7675
CAS No.:251908-92-6
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
- Fmoc-D-Threoninol
Catalog No.:BCC2702
CAS No.:252049-02-8
- Fmoc-Lys(Me2)-OH
Catalog No.:BCC2567
CAS No.:252049-10-8
HDAC inhibitor M344 suppresses MCF-7 breast cancer cell proliferation.[Pubmed:22436652]
Biomed Pharmacother. 2012 Apr;66(3):232-6.
Histone deacetylase (HDAC) inhibitors represent a novel class of drugs that selectively induce cell cycle arrest and apoptosis in transformed cells. This study examined, for the first time, the effects of the relatively new HDAC inhibitor, M344 [4-dimethylamino-N-(6-hydroxycarbamoylhexyl)-benzamide], on the proliferation of MCF-7 breast cancer cells. MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assays revealed significant concentration- and time-dependent decreases in MCF-7 cell proliferation following treatment with M344 (1-100muM). In contrast to the significant induction of p21(waf1/cip1) mRNA expression following treatment with M344 (10muM) for 1 or 3 days, there was a significant decrease in p53 mRNA expression, although p53 protein levels were unchanged. Similar treatment with M344 also induced expression of the pro-apoptotic genes, Puma and Bax, together with the morphological features of apoptosis, in MCF-7 cells. The results of this study reinforce previous findings indicating that HDAC inhibitors are an important group of oncostatic drugs, and show that M344 is a potent suppressor of breast cancer cell proliferation.
The effect of the histone deacetylase inhibitor M344 on BRCA1 expression in breast and ovarian cancer cells.[Pubmed:21854619]
Cancer Cell Int. 2011 Aug 19;11(1):29.
BACKGROUND: The inhibition of Breast Cancer 1 (BRCA1) expression sensitizes breast and ovarian cancer cells to platinum chemotherapy. However, therapeutically relevant agents that target BRCA1 expression have not been identified. Our recent report suggested the potential of the histone deacetylase (HDAC) inhibitor, M344, to inhibit BRCA1 expression. In this study, we further evaluated the effect of M344 on BRCA1 mRNA and protein expression, as well as its effect on cisplatin-induced cytotoxicity in various breast (MCF7, T-47D and HCC1937) and ovarian (A2780s, A2780cp and OVCAR-4) cancer cell lines. RESULTS: With the addition of M344, the platinum-sensitive breast and ovarian cancer cell lines that displayed relatively high BRCA1 protein levels demonstrated significant potentiation of cisplatin cytotoxicity in association with a reduction of BRCA1 protein. The cisplatin-resistant cell lines, T-47D and A2780s, elicited increased cytotoxicity of cisplatin with M344 and down regulation of BRCA1 protein levels. A2780s cells subjected to combination platinum and M344 treatment, demonstrated increased DNA damage as assessed by the presence of phosphorylated H2A.X foci in comparison to either treatment alone. Using Chromatin Immunoprecipitation, A2780s and MCF7 cells exposed to M344 alone and in combination with cisplatin, did not demonstrate enhanced acetylated Histone 4 at the BRCA1 promoter, suggesting an indirect effect on this promoter. CONCLUSIONS: The enhanced sensitivity of HDAC inhibition to platinum may be mediated through a BRCA1-dependent mechanism in breast and ovarian cancer cells. The findings of this study may be important in the future design of clinical trials involving HDAC inhibitors using BRCA1 as a tumour biomarker.
Selective histonedeacetylase inhibitor M344 intervenes in HIV-1 latency through increasing histone acetylation and activation of NF-kappaB.[Pubmed:23166597]
PLoS One. 2012;7(11):e48832.
BACKGROUND: Histone deacetylase (HDAC) inhibitors present an exciting new approach to activate HIV production from latently infected cells to potentially enhance elimination of these cells and achieve a cure. M344, a novel HDAC inhibitor, shows robust activity in a variety of cancer cells and relatively low toxicity compared to trichostatin A (TSA). However, little is known about the effects and action mechanism of M344 in inducing HIV expression in latently infected cells. METHODOLOGY/PRINCIPAL FINDINGS: Using the Jurkat T cell model of HIV latency, we demonstrate that M344 effectively reactivates HIV-1 gene expression in latently infected cells. Moreover, M344-mediated activation of the latent HIV LTR can be strongly inhibited by a NF-kappaB inhibitor aspirin. We further show that M344 acts by increasing the acetylation of histone H3 and histone H4 at the nucleosome 1 (nuc-1) site of the HIV-1 long terminal repeat (LTR) and by inducing NF-kappaB p65 nuclear translocation and direct RelA DNA binding at the nuc-1 region of the HIV-1 LTR. We also found that M344 synergized with prostratin to activate the HIV-1 LTR promoter in latently infected cells. CONCLUSIONS/SIGNIFICANCE: These results suggest the potential of M344 in anti-latency therapies and an important role for histone modifications and NF-kappaB transcription factors in regulating HIV-1 LTR gene expression.
The benzamide M344, a novel histone deacetylase inhibitor, significantly increases SMN2 RNA/protein levels in spinal muscular atrophy cells.[Pubmed:16724231]
Hum Genet. 2006 Aug;120(1):101-10.
Proximal spinal muscular atrophy (SMA) is a common autosomal recessively inherited neuromuscular disorder causing infant death in half of all patients. Homozygous loss of the survival motor neuron 1 (SMN1) gene causes SMA, whereas the number of the SMN2 copy genes modulates the severity of the disease. Due to a silent mutation within an exonic splicing enhancer, SMN2 mainly produces alternatively spliced transcripts lacking exon 7 and only approximately 10% of a full-length protein identical to SMN1. However, SMN2 represents a promising target for an SMA therapy. The correct splicing of SMN2 can be efficiently restored by over-expression of the splicing factor Htra2-beta1 as well as by exogenous factors like drugs that inhibit histone deacetylases (HDACs). Here we show that the novel benzamide M344, an HDAC inhibitor, up-regulates SMN2 protein expression in fibroblast cells derived from SMA patients up to 7-fold after 64 h of treatment. Moreover, M344 significantly raises the total number of gems/nucleus as well as the number of nuclei that contain gems. This is the strongest in vitro effect of a drug on the SMN protein level reported so far. The reversion of Delta7-SMN2 into FL-SMN2 transcripts as demonstrated by quantitative RT-PCR is most likely facilitated by elevated levels of Htra2-beta1. Investigations of the cytotoxicity of M344 using an MTT assay revealed toxic cell effects only at very high concentrations. In conclusion, M344 can be considered as highly potent HDAC inhibitor which is active at low doses and therefore represents a promising candidate for a causal therapy of SMA.
M344 is a novel synthesized histone deacetylase inhibitor that induces growth inhibition, cell cycle arrest, and apoptosis in human endometrial cancer and ovarian cancer cells.[Pubmed:16263156]
Gynecol Oncol. 2006 Apr;101(1):108-13.
OBJECTIVE: Histone deacetylase inhibitors (HDACIs) can inhibit cell proliferation, induce cell cycle arrest, and stimulate apoptosis of cancer cells. METHODS: We investigated the effects of a novel synthesized HDACI, M344, on Ishikawa endometrial cancer cell line, SK-OV-3 ovarian cancer cell line, and normal human endometrial epithelial cells. Endometrial and ovarian cancer cells were treated with various concentrations of M344, and its effect on cell growth, cell cycle, apoptosis, and related measurements was investigated. RESULTS: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays showed that all endometrial and ovarian cancer cell lines were sensitive to the growth inhibitory effect of M344, although normal endometrial epithelial cells were viable after the treatment with the same doses of M344 that induced growth inhibition of endometrial and ovarian cancer cells. Cell cycle analysis indicated that their exposure to M344 decreased the proportion of cells in the S-phase and increased the proportion in the G0/G1 phases of the cell cycle. Induction of apoptosis was confirmed by annexin V staining of externalized phosphatidylserine and loss of the transmembrane potential of mitochondria. This induction occurred in concert with altered expression of genes related to cell growth, malignant phenotype, and apoptosis. Furthermore, M344 treatment of these cell lines increased acetylation of H3 and H4 histone tails. CONCLUSIONS: These results raise the possibility that M344 may prove particularly effective in the treatment of endometrial cancers and ovarian cancers.
Amide analogues of trichostatin A as inhibitors of histone deacetylase and inducers of terminal cell differentiation.[Pubmed:10579829]
J Med Chem. 1999 Nov 4;42(22):4669-79.
Inhibitors of histone deacetylase (HD) bear great potential as new drugs due to their ability to modulate transcription and to induce apoptosis or differentiation in cancer cells. We have described previously analogues of the complex natural HD inhibitors trapoxin B and trichostatin A with activities in the submicromolar range. Here we report structure-activity relationship analyses of further analogues of trichostatin A with respect to in vitro inhibition of maize HD-2 and their ability to induce terminal cell differentiation in Friend leukemic cells. This is the first report that shows the correlation between HD inhibitory activity and action on cancer cells on a larger series of similar compounds. Only the compounds that inhibit HD induce differentiation and/or exert antiproliferative activities in cell culture. Our studies support the use of in vitro systems as screening tools and provide structure-activity relationships that merit further investigation of this interesting target.


