LolineCAS# 25161-91-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 25161-91-5 | SDF | Download SDF |
| PubChem ID | 716098 | Appearance | Powder |
| Formula | C8H14N2O | M.Wt | 154.21 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Synonyms | Festucine | ||
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
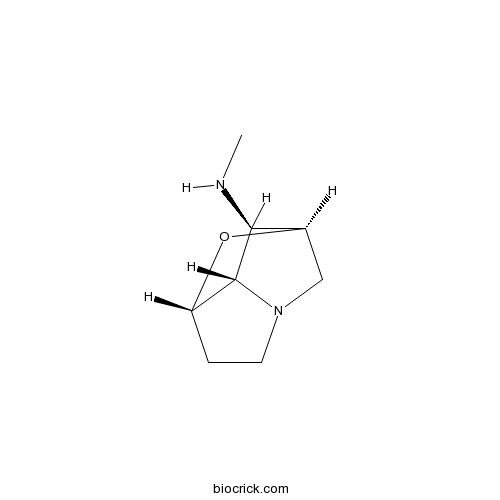
| Chemical Name | (1R,3S,7S,8R)-N-methyl-2-oxa-6-azatricyclo[4.2.1.03,7]nonan-8-amine | ||
| SMILES | CNC1C2CN3C1C(O2)CC3 | ||
| Standard InChIKey | OPMNROCQHKJDAQ-FKSUSPILSA-N | ||
| Standard InChI | InChI=1S/C8H14N2O/c1-9-7-6-4-10-3-2-5(11-6)8(7)10/h5-9H,2-4H2,1H3/t5-,6+,7-,8+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Loline can reduce prolactin release at the highest concentration, 10(-4) M. |

Loline Dilution Calculator

Loline Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4847 mL | 32.4233 mL | 64.8466 mL | 129.6933 mL | 162.1166 mL |
| 5 mM | 1.2969 mL | 6.4847 mL | 12.9693 mL | 25.9387 mL | 32.4233 mL |
| 10 mM | 0.6485 mL | 3.2423 mL | 6.4847 mL | 12.9693 mL | 16.2117 mL |
| 50 mM | 0.1297 mL | 0.6485 mL | 1.2969 mL | 2.5939 mL | 3.2423 mL |
| 100 mM | 0.0648 mL | 0.3242 mL | 0.6485 mL | 1.2969 mL | 1.6212 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Acevaltrate
Catalog No.:BCN7127
CAS No.:25161-41-5
- 5-Chloro-2-nitrobenzoic acid
Catalog No.:BCC8743
CAS No.:2516-95-2
- Tesaglitazar
Catalog No.:BCC7828
CAS No.:251565-85-2
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- SU 16f
Catalog No.:BCC7639
CAS No.:251356-45-3
- Urotensin II (human)
Catalog No.:BCC5796
CAS No.:251293-28-4
- Crebanine
Catalog No.:BCN5117
CAS No.:25127-29-1
- FRATide
Catalog No.:BCC5821
CAS No.:251087-38-4
- Antazoline HCl
Catalog No.:BCC4627
CAS No.:2508-72-7
- Salvisyrianone
Catalog No.:BCN4821
CAS No.:250691-57-7
- NNC 63-0532
Catalog No.:BCC7177
CAS No.:250685-44-0
- Pedunsaponin A
Catalog No.:BCN8192
CAS No.:250613-27-5
- Isohomoarbutin
Catalog No.:BCN7612
CAS No.:25162-30-5
- Dynamin inhibitory peptide
Catalog No.:BCC1034
CAS No.:251634-21-6
- AM 1172
Catalog No.:BCC7675
CAS No.:251908-92-6
- SLV 320
Catalog No.:BCC7656
CAS No.:251945-92-3
- A 205804
Catalog No.:BCC3944
CAS No.:251992-66-2
- AZD7545
Catalog No.:BCC4294
CAS No.:252017-04-2
- Fmoc-D-Threoninol
Catalog No.:BCC2702
CAS No.:252049-02-8
- Fmoc-Lys(Me2)-OH
Catalog No.:BCC2567
CAS No.:252049-10-8
- Tertiapin-Q
Catalog No.:BCC5740
CAS No.:252198-49-5
- 1-Cinnamoylpyrrole
Catalog No.:BCN4006
CAS No.:252248-89-8
- 9,9'-O-Isopropyllidene-isolariciresinol
Catalog No.:BCN1474
CAS No.:252333-71-4
- Isotaxiresinol 9,9'-acetonide
Catalog No.:BCN4663
CAS No.:252333-72-5
Loline alkaloid production by fungal endophytes of Fescue species select for particular epiphytic bacterial microflora.[Pubmed:24108329]
ISME J. 2014 Feb;8(2):359-68.
The leaves of fescue grasses are protected from herbivores by the production of Loline alkaloids by the mutualist fungal endophytes Neotyphodium sp. or Epichloe sp. Most bacteria that reside on the leaf surface of such grasses can consume these defensive chemicals. Loline-consuming bacteria are rare on the leaves of other plant species. Several bacterial species including Burkholderia ambifaria recovered from tall fescue could use N-formyl Loline as a sole carbon and nitrogen source in culture and achieved population sizes that were about eightfold higher when inoculated onto plants harboring Loline-producing fungal endophytes than on plants lacking such endophytes or which were colonized by fungal variants incapable of Loline production. In contrast, mutants of B. ambifaria and other bacterial species incapable of Loline catabolism achieved similarly low population sizes on tall fescue colonized by Loline-producing Neotyphodium sp. and on plants lacking this endophytic fungus. Lolines that are released onto the surface of plants benefiting from a fungal mutualism thus appear to be a major resource that can be exploited by epiphytic bacteria, thereby driving the establishment of a characteristic bacterial community on such plants.
An efficient synthesis of loline alkaloids.[Pubmed:21697875]
Nat Chem. 2011 Jun 19;3(7):543-5.
Loline (1) is a small alkaloid that, in spite of its simple-looking structure, has posed surprising challenges to synthetic chemists. It has been known for more than a century and has been the subject of extensive biological investigations, but only two total syntheses have been achieved to date. Here, we report an asymmetric total synthesis of Loline that, with less then ten steps, is remarkably short. Our synthesis incorporates a Sharpless epoxidation, a Grubbs olefin metathesis and an unprecedented transannular aminobromination, which converts an eight-membered cyclic carbamate into a bromopyrrolizidine. The synthesis is marked by a high degree of chemo- and stereoselectivity and gives access to several members of the Loline alkaloid family. It delivers sufficient material to support a programme aimed at studying the complex interactions between plants, fungi, insects and bacteria brokered by Loline alkaloids.
Excretion of loline alkaloids in urine and faeces of sheep dosed with meadow fescue (Festuca pratensis) seed containing high concentrations of loline alkaloids.[Pubmed:22480356]
N Z Vet J. 2012 May;60(3):176-82.
AIM: To determine the effect of oral dosing of sheep with Loline alkaloids on their excretion in urine and faeces, and to monitor for any toxic effects. METHODS: In Experiment 1, six 9-month-old ewe lambs were given a single oral dose of Loline alkaloids (52 mg/kg bodyweight (BW); acute exposure) as a suspension of ground meadow fescue (Festuca pratensis) seed in water. In Experiment 2, on six consecutive days, six ewe lambs were given three doses of Loline (68 mg/kg BW/day; chronic exposure). Blood was collected at variable intervals up to 72 h in Experiment 1, and up to 8 days in Experiment 2, for haematology and measurement of alkaline phosphatase, aspartate aminotransaminase, creatine kinase and gamma-glutamyl transferase in plasma. Urine and faecal samples were collected at similar times for measurement of creatinine in urine and Loline alkaloid analysis. A post mortem with histopathology was carried out on two animals at the end of each experiment. RESULTS: The Loline alkaloids, N-acetyl norLoline, N-formyl Loline, N-acetyl Loline, N-methyl Loline and Loline base were detected in urine within 15 minutes after the single dosing. N-formyl Loline and Loline base were the predominant metabolites in urine in both experiments. The total quantity of Lolines excreted in both urine and faeces was 10% and 4% of the amount dosed in Experiments 1 and 2, respectively. In both experiments, the clinical chemistry parameters in blood and urine were within normal ranges. Post-mortem and histopathological examination did not show any abnormalities. CONCLUSIONS: This is the first report of Loline alkaloid profiles in both urine and faeces of sheep. The appearance of Loline alkaloids and the Loline base in urine within 15 minutes suggests rapid uptake, metabolism and excretion. Loline alkaloids were non-toxic to sheep at the concentrations they are exposed to under New Zealand grazing conditions. The low recovery of Loline alkaloids in urine and faeces in the absence of toxicity signs suggests Lolines are extensively metabolised; probably to forms other than N-formyl Loline, N-methyl Loline, N-acetyl Loline, N-acetyl norLoline, and Loline base in the digestive tract of sheep prior to absorption, and/or in the liver or other tissues following absorption.


