Ezatiostat hydrochlorideGST inhibitor CAS# 286942-97-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 286942-97-0 | SDF | Download SDF |
| PubChem ID | 25063260 | Appearance | Powder |
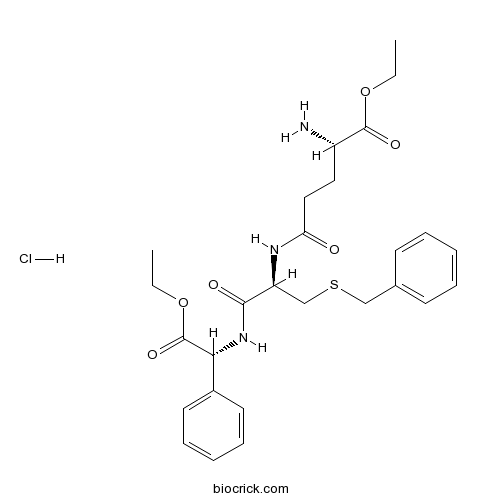
| Formula | C27H36ClN3O6S | M.Wt | 566.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TER199; TLK199 | ||
| Solubility | >28.3mg/mL in DMSO | ||
| Chemical Name | ethyl (2S)-2-amino-5-[[(2R)-3-benzylsulfanyl-1-[[(1R)-2-ethoxy-2-oxo-1-phenylethyl]amino]-1-oxopropan-2-yl]amino]-5-oxopentanoate;hydrochloride | ||
| SMILES | CCOC(=O)C(CCC(=O)NC(CSCC1=CC=CC=C1)C(=O)NC(C2=CC=CC=C2)C(=O)OCC)N.Cl | ||
| Standard InChIKey | XJDYQYNYISTAMO-GFDYFVENSA-N | ||
| Standard InChI | InChI=1S/C27H35N3O6S.ClH/c1-3-35-26(33)21(28)15-16-23(31)29-22(18-37-17-19-11-7-5-8-12-19)25(32)30-24(27(34)36-4-2)20-13-9-6-10-14-20;/h5-14,21-22,24H,3-4,15-18,28H2,1-2H3,(H,29,31)(H,30,32);1H/t21-,22-,24+;/m0./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ezatiostat hydrochloride is a glutathione analog inhibitor of glutathione S-transferase P1-1 (GSTP1-1).In Vitro:Ezatiostat causes dissociation of the enzyme from the jun-N-terminal kinase/c-Jun (JNK/JUN) complex, leading to JNK activation by phosphorylation. The therapeutic action of ezatiostat appears to include both proliferation of normal myeloid progenitors as well as apoptosis of the malignant clone[1]. References: | |||||

Ezatiostat hydrochloride Dilution Calculator

Ezatiostat hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7664 mL | 8.8322 mL | 17.6644 mL | 35.3288 mL | 44.161 mL |
| 5 mM | 0.3533 mL | 1.7664 mL | 3.5329 mL | 7.0658 mL | 8.8322 mL |
| 10 mM | 0.1766 mL | 0.8832 mL | 1.7664 mL | 3.5329 mL | 4.4161 mL |
| 50 mM | 0.0353 mL | 0.1766 mL | 0.3533 mL | 0.7066 mL | 0.8832 mL |
| 100 mM | 0.0177 mL | 0.0883 mL | 0.1766 mL | 0.3533 mL | 0.4416 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ezatiostat hydrochloride(TLK199) is an effective inhibitor of glutathione S-transferase (GST)[1].
Ezatiostat hydrochloride (TLK199) is a novel glutathione analog and the potential treatment of cytopenias. In addition, Ezatiostat hydrochloride has been revealed to selectively bind to and thus inhibit GST P1-1. Because GST P1-1 can bind to and inhibit JNK, Ezatiostat hydrochloride has also been exhibited to inhibit GST P1-1, activate JNK, and promote the growth and maturation of hematopoietic progenitors in preclinical models. Moreover, Ezatiostat hydrochloride has been reported to stimulate the proliferation of myeloid precursors. Ezatiostat hydrochloride has been elucidated to induce growth inhibition and cellular apoptosis in human leukemia cells (HL-60) with a CC50 value of 6-17μM. Apart from these, Ezatiostat hydrochloride has shown the stimulation of multilineage differentiation in mature monocytes, granulocytes and erythrocytes [1,2].
References:
[1] Tew KD1, Dutta S, Schultz M.Inhibitors of glutathione S-transferases as therapeutic agents. Adv Drug Deliv Rev. 1997 Jul 7;26(2-3):91-104.
[2] Raza A1, Galili N, Callander N, Ochoa L, Piro L, Emanuel P, Williams S, Burris H 3rd, Faderl S, Estrov Z, Curtin P, Larson RA, Keck JG, Jones M, Meng L, Brown GL. Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome. J Hematol Oncol. 2009 May 13;2:20.
- Fesoterodine Fumarate
Catalog No.:BCC4584
CAS No.:286930-03-8
- 6'-Amino-3',4'-(methylenedioxy)acetophenone
Catalog No.:BCC8760
CAS No.:28657-75-2
- Epoxylathyrol
Catalog No.:BCN5382
CAS No.:28649-60-7
- Euphorbiasteroid
Catalog No.:BCN2781
CAS No.:28649-59-4
- Multicaulisin
Catalog No.:BCN7840
CAS No.:286461-76-5
- L-838,417
Catalog No.:BCC7617
CAS No.:286456-42-6
- Meloscandonine
Catalog No.:BCN5186
CAS No.:28645-27-4
- Nigericin sodium salt
Catalog No.:BCC7915
CAS No.:28643-80-3
- KRN 633
Catalog No.:BCC2544
CAS No.:286370-15-8
- S 14506 hydrochloride
Catalog No.:BCC7174
CAS No.:286369-38-8
- Erythristemine
Catalog No.:BCN5184
CAS No.:28619-41-2
- 8-Prenylkaempferol
Catalog No.:BCN3311
CAS No.:28610-31-3
- NCX 4040
Catalog No.:BCC7944
CAS No.:287118-97-2
- PD 180970
Catalog No.:BCC3894
CAS No.:287204-45-9
- MA 2029
Catalog No.:BCC7983
CAS No.:287206-61-5
- Oxcarbazepine
Catalog No.:BCC5077
CAS No.:28721-07-5
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- 3CAI
Catalog No.:BCC5402
CAS No.:28755-03-5
- Apigenin 5-O-beta-D-glucopyranoside
Catalog No.:BCN5185
CAS No.:28757-27-9
- Rosuvastatin
Catalog No.:BCC4139
CAS No.:287714-41-4
- 4,5,6,7-Tetrahydrothieno [3,2,c]pyridine hydrochloride
Catalog No.:BCC8664
CAS No.:28783-41-7
- Nordihydrocapsaicin
Catalog No.:BCN2387
CAS No.:28789-35-7
- H-Cys(Acm)-OH.HCl
Catalog No.:BCC2903
CAS No.:28798-28-9
- Peptone, bacteriological
Catalog No.:BCC1210
CAS No.:288-88-0
Phase 1 dose-ranging study of ezatiostat hydrochloride in combination with lenalidomide in patients with non-deletion (5q) low to intermediate-1 risk myelodysplastic syndrome (MDS).[Pubmed:22546242]
J Hematol Oncol. 2012 Apr 30;5:18.
BACKGROUND: Ezatiostat, a glutathione S-transferase P1-1 inhibitor, promotes the maturation of hematopoietic progenitors and induces apoptosis in cancer cells. RESULTS: Ezatiostat was administered to 19 patients with non-deletion(5q) myelodysplastic syndrome (MDS) at one of two doses (2000 mg or 2500 mg/day) in combination with 10 mg of lenalidomide on days 1-21 of a 28-day cycle. No unexpected toxicities occurred and the incidence and severity of adverse events (AEs) were consistent with that expected for each drug alone. The most common non-hematologic AEs related to ezatiostat in combination with lenalidomide were mostly grade 1 and 2 fatigue, anorexia, nausea, diarrhea, and vomiting; hematologic AEs due to lenalidomide were thrombocytopenia, neutropenia, and anemia. One of 4 evaluable patients (25%) in the 2500/10 mg dose group experienced an erythroid hematologic improvement (HI-E) response by 2006 MDS International Working Group (IWG) criteria. Four of 10 evaluable patients (40%) in the 2000 mg/10 mg dose group experienced an HI-E response. Three of 7 (43%) red blood cell (RBC) transfusion-dependent patients became RBC transfusion independent, including one patient for whom prior lenalidomide monotherapy was ineffective. Three of 5 (60%) thrombocytopenic patients had an HI-platelet (HI-P) response. Bilineage HI-E and HI-P responses occurred in 3 of 5 (60%), 1 of 3 with HI-E and HI-N (33%), and 1 of 3 with HI-N and HI-P (33%). One of 3 patients (33%) with pancytopenia experienced a complete trilineage response. All multilineage responses were observed in the 2000/10 mg doses recommended for future studies. CONCLUSIONS: The tolerability and activity profile of ezatiostat co-administered with lenalidomide supports the further development of ezatiostat in combination with lenalidomide in MDS and also encourages studies of this combination in other hematologic malignancies where lenalidomide is active.
Phase 1-2a multicenter dose-escalation study of ezatiostat hydrochloride liposomes for injection (Telintra, TLK199), a novel glutathione analog prodrug in patients with myelodysplastic syndrome.[Pubmed:19439093]
J Hematol Oncol. 2009 May 13;2:20.
BACKGROUND: Ezatiostat hydrochloride liposomes for injection, a glutathione S-transferase P1-1 inhibitor, was evaluated in myelodysplastic syndrome (MDS). The objectives were to determine the safety, pharmacokinetics, and hematologic improvement (HI) rate. Phase 1-2a testing of ezatiostat for the treatment of MDS was conducted in a multidose-escalation, multicenter study. Phase 1 patients received ezatiostat at 5 dose levels (50, 100, 200, 400 and 600 mg/m2) intravenously (IV) on days 1 to 5 of a 14-day cycle until MDS progression or unacceptable toxicity. In phase 2, ezatiostat was administered on 2 dose schedules: 600 mg/m2 IV on days 1 to 5 or days 1 to 3 of a 21-day treatment cycle. RESULTS: 54 patients with histologically confirmed MDS were enrolled. The most common adverse events were grade 1 or 2, respectively, chills (11%, 9%), back pain (15%, 2%), flushing (19%, 0%), nausea (15%, 0%), bone pain (6%, 6%), fatigue (0%, 13%), extremity pain (7%, 4%), dyspnea (9%, 4%), and diarrhea (7%, 4%) related to acute infusional hypersensitivity reactions. The concentration of the primary active metabolites increased proportionate to ezatiostat dosage. Trilineage responses were observed in 4 of 16 patients (25%) with trilineage cytopenia. Hematologic Improvement-Erythroid (HI-E) was observed in 9 of 38 patients (24%), HI-Neutrophil in 11 of 26 patients (42%) and HI-Platelet in 12 of 24 patients (50%). These responses were accompanied by improvement in clinical symptoms and reductions in transfusion requirements. Improvement in bone marrow maturation and cellularity was also observed. CONCLUSION: Phase 2 studies of Ezatiostat hydrochloride liposomes for injection in MDS are supported by the tolerability and HI responses observed. An oral formulation of Ezatiostat hydrochloride tablets is also in phase 2 clinical development. TRIAL REGISTRATION: Clinicaltrials.gov: NCT00035867.
Ezatiostat hydrochloride for the treatment of myelodysplastic syndromes.[Pubmed:25724698]
Expert Opin Investig Drugs. 2015 May;24(5):725-33.
INTRODUCTION: Myelodysplastic syndromes (MDSs) are associated with significant morbidity due to ineffective hematopoiesis. Given the limited number of drugs approved by the FDA, there is a need for new therapeutic options. Ezatiostat is a novel agent targeting oxidative stress via inhibition of glutathione S-transferase 1. AREAS COVERED: Herein, the authors summarize the standard of care in order to build the framework for therapeutic advancements. The purpose of this paper is to review the body of preclinical and clinical research literature on the investigational agent Ezatiostat hydrochloride (TLK199) for the treatment of MDSs. The article includes details of the pathophysiology, pharmacology, toxicity and efficacy of Ezatiostat hydrochloride from controlled studies in patients with myelodysplasia. EXPERT OPINION: MDS clonal heterogeneity and clonal architecture complexity has presented a significant technical challenge in developing effective therapies. Ezatiostat offers a unique and specific mechanism to improve the transfusion burden associated with myelodysplasia. Since it is tolerable as a monotherapy, combining ezatiostat with agents such as lenalidomide may have the most potential benefit.
Phase 1 multicenter dose-escalation study of ezatiostat hydrochloride (TLK199 tablets), a novel glutathione analog prodrug, in patients with myelodysplastic syndrome.[Pubmed:19398716]
Blood. 2009 Jun 25;113(26):6533-40.
Phase 1 testing of ezatiostat, a glutathione S-transferase P1-1 inhibitor, for the treatment of myelodysplastic syndrome was conducted in a multidose-escalation study. Patients received 10 dose levels (200, 400, 1000, 1400, 2000, 2400, 3000, 4000, 5000, and 6000 mg) of ezatiostat tablets in divided doses on days 1 to 7 of a 21-day cycle for a maximum of 8 cycles. The safety and pharmacokinetics of ezatiostat were evaluated. Forty-five patients with low to intermediate-2 International Prognostic Scoring System risk myelodysplastic syndrome were enrolled. No dose-limiting toxicities were observed. The most common grade 1 or 2, respectively, treatment-related adverse events were nonhematologic: nausea (56%, 9%), diarrhea (36%, 7%), vomiting (24%, 7%), abdominal pain (9%, 0%), constipation (4%, 9%), anorexia (3%, 7%), and dyspepsia (3%, 7%). Concentration of the primary active metabolite, TLK236, increased proportionate to ezatiostat dosage. Seventeen hematologic improvement (HI) responses by International Working Group criteria were observed at dose levels of 200 to 6000 mg/day with 11 HI responses at doses of 4000 to 6000 mg/day. HI responses occurred in all lineages including 3 bilineage and 1 complete cytogenetic response. Decreased number of red blood cell and platelet transfusions and in some cases transfusion independence were attained. Extended dose schedules of ezatiostat tablets are under investigation.


