SP 600125JNK1/2/3 inhibitor CAS# 129-56-6 |
- JNK-IN-7
Catalog No.:BCC1672
CAS No.:1408064-71-0
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- CEP 1347
Catalog No.:BCC7982
CAS No.:156177-65-0
- AEG 3482
Catalog No.:BCC8088
CAS No.:63735-71-7
- AS 602801
Catalog No.:BCC1369
CAS No.:848344-36-5
- CC-930
Catalog No.:BCC1459
CAS No.:899805-25-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

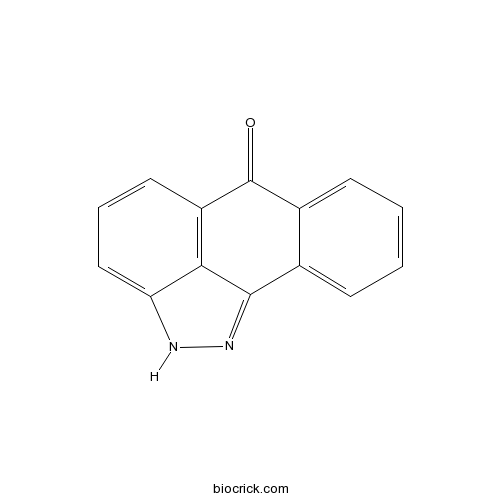
| Cas No. | 129-56-6 | SDF | Download SDF |
| PubChem ID | 8515 | Appearance | Powder |
| Formula | C14H8N2O | M.Wt | 220.23 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in ethanol with gentle warming and to 100 mM in DMSO | ||
| SMILES | C1=CC=C2C(=C1)C3=NNC4=CC=CC(=C43)C2=O | ||
| Standard InChIKey | ACPOUJIDANTYHO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C14H8N2O/c17-14-9-5-2-1-4-8(9)13-12-10(14)6-3-7-11(12)15-16-13/h1-7H,(H,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective JNK inhibitor. Competitively and reversibly inhibits JNK1, 2 and 3 (IC50 = 40 - 90 nM) with negligible activity at ERK2, p38β and a range of enzymes. Active in vivo. Exhibits reduced selectivity over other protein kinases under certain conditions. Protects renal tubular epithelial cells against ischemia/reperfusion-induced apoptosis. Prevents BMP9-induced osteogenic differentiation of MSCs. Essential component of medium for maintaining stem cells in naive pluripotent state. Available as part of the MAPK Inhibitor. |

SP 600125 Dilution Calculator

SP 600125 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5407 mL | 22.7035 mL | 45.4071 mL | 90.8141 mL | 113.5177 mL |
| 5 mM | 0.9081 mL | 4.5407 mL | 9.0814 mL | 18.1628 mL | 22.7035 mL |
| 10 mM | 0.4541 mL | 2.2704 mL | 4.5407 mL | 9.0814 mL | 11.3518 mL |
| 50 mM | 0.0908 mL | 0.4541 mL | 0.9081 mL | 1.8163 mL | 2.2704 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4541 mL | 0.9081 mL | 1.1352 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
SP600125 is a selective, reversible and ATP-competitive inhibitor of Jun N-terminal kinase (JNK) with IC50 values of 40, 40 and 90 nM for JNK1, 2 and 3, respectively [1].
SP600125 was screened out from a time-resolved f luorescence assay using the GST-c-Jun and recombinant human JNK2. In this assay, SP600125 showed a Ki value of 190 nM. SP600125 was also found to inhibit JNK1, 2 and 3 isoforms in the selectivity tests. The selectivity of SP600125 for JNK is 300-fold greater than that for ERK1 and p38-2. In Jurkat T cells, SP600125 suppressed the phosphorylation of c-Jun with IC50 of 5-10 μM. SP600125 also inhibited the expression of IL-2 and IFN-γ in cells stimulated with PMA and phytohemagglutinin, since JNK had been reported to regulate the transcription of IL-2. Besides that, SP600125 exerted differential inhibition of cytokines in CD4+ cells as well as inflammatory genes in monocytes. Moreover, SP600125 administration significantly inhibited TNF-α expression induced by LPS in a mouse model, suggesting that it had efficacy in endotoxin-induced inf lammation in vivo [1].
References:
[1] Bennett B L, Sasaki D T, Murray B W, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proceedings of the National Academy of Sciences, 2001, 98(24): 13681-13686.
- Methysergide maleate
Catalog No.:BCC5698
CAS No.:129-49-7
- Suramin hexasodium salt
Catalog No.:BCC7079
CAS No.:129-46-4
- Maohuoside A
Catalog No.:BCN5348
CAS No.:128988-55-6
- Fargesol
Catalog No.:BCN6421
CAS No.:128855-64-1
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- FRAX597
Catalog No.:BCC4172
CAS No.:1286739-19-2
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- Buclizine HCl
Catalog No.:BCC4516
CAS No.:129-74-8
- Rivastigmine Tartrate
Catalog No.:BCC3851
CAS No.:129101-54-8
- Evodosin A
Catalog No.:BCN7322
CAS No.:1291053-38-7
- ENMD-2076 L-(+)-Tartaric acid
Catalog No.:BCC2185
CAS No.:1291074-87-7
- CGRP 8-37 (rat)
Catalog No.:BCC5717
CAS No.:129121-73-9
- 2-(2,2-Dimethyl-1,3-dioxolan-4-yl)propane-1,2-diol
Catalog No.:BCC8475
CAS No.:129141-48-6
- Gancaonin M
Catalog No.:BCN4757
CAS No.:129145-51-3
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- Iberiotoxin
Catalog No.:BCC6932
CAS No.:129203-60-7
- (2S,3S)-(-)-Glucodistylin
Catalog No.:BCN6156
CAS No.:129212-92-6
- 15-Dihydroepioxylubimin
Catalog No.:BCN4800
CAS No.:129214-59-1
- IKKε-IN-1
Catalog No.:BCC5514
CAS No.:1292310-49-6
Induction of apoptosis and cell cycle arrest by a specific c-Jun NH2-terminal kinase (JNK) inhibitor, SP-600125, in gastrointestinal cancers.[Pubmed:16337741]
Cancer Lett. 2006 Sep 28;241(2):268-74.
The c-Jun NH(2)-terminal kinase (JNK) is activated in several tumor cell lines. The aim of this study was to determine the effects of SP-600125, a specific JNK inhibitor, on the viability, apoptosis, cell cycle distribution of gastrointestinal cancer cells, and the potential anti-tumor mechanisms. Three gastric cancer cell lines, AGS, BCG-823 and MKN-45, and three colorectal cancer cell lines, SW1116, COLO205 and HT-29, were used. Cells were treated with SP-600125, and cell viability, apoptosis and cell cycle distribution, caspase-3 activity, expression of JNK and apoptosis related proteins were detected. SP-600125 inhibited cell proliferation by 10-80% for the different cell lines, and increased apoptosis by 1.5-4.5 folds for COLO205, BCG-823, MKN-45, AGS cells. Caspase-8 and caspase-3 were involved in the induction of apoptosis. SP-600125 caused G2/M cell cycle arrest and elevation of cyclin B1 and p27(kip). The differential response in cells to SP-600125 was associated with the basal level of phosphorylated JNK2. It is concluded that SP-600125 inhibits proliferation, induces apoptosis and causes cell cycle arrest in gastrointestinal cancer cells, indicating that JNK inhibitors have an anti-tumor effect and are potential therapeutic agents for cancers.
Effects of the JNK inhibitor anthra[1,9-cd]pyrazol-6(2H)-one (SP-600125) on soluble guanylyl cyclase alpha1 gene regulation and cGMP synthesis.[Pubmed:15888553]
Am J Physiol Cell Physiol. 2005 Oct;289(4):C778-84.
The decreased expression of the nitric oxide (NO) receptor, soluble guanylyl cyclase (sGC), occurs in response to multiple stimuli in vivo and in cell culture and correlates with various disease states such as hypertension, inflammation, and neurodegenerative disorders. The ability to understand and modulate sGC expression and cGMP levels in any of these conditions could be a valuable therapeutic tool. We demonstrate herein that the c-Jun NH2-terminal kinase JNK II inhibitor anthra[1,9-cd]pyrazol-6(2H)-one (SP-600125) completely blocked the decreased expression of sGCalpha1-subunit mRNA by nerve growth factor (NGF) in PC12 cells. Inhibitors of the ERK and p38 MAPK pathways, PD-98059 and SB-203580, had no effect. SP-600125 also inhibited the NGF-mediated decrease in the expression of sGCalpha1 protein as well as sGC activity in PC12 cells. Other experiments revealed that decreased sGCalpha1 mRNA expression through a cAMP-mediated pathway, using forskolin, was not blocked by SP-600125. We also demonstrate that TNF-alpha/IL-1beta stimulation of rat fetal lung (RFL-6) fibroblast cells resulted in sGCalpha1 mRNA inhibition, which was blocked by SP-600125. Expression of a constitutively active JNKK2-JNK1 fusion protein in RFL-6 cells caused endogenous sGCalpha1 mRNA levels to decrease, while a constitutively active ERK2 protein had no effect. Collectively, these data demonstrate that SP-600125 may influence the intracellular levels of the sGCalpha1-subunit in certain cell types and may implicate a role for c-Jun kinase in the regulation of sGCalpha1 expression.
Derivation of novel human ground state naive pluripotent stem cells.[Pubmed:24172903]
Nature. 2013 Dec 12;504(7479):282-6.
Mouse embryonic stem (ES) cells are isolated from the inner cell mass of blastocysts, and can be preserved in vitro in a naive inner-cell-mass-like configuration by providing exogenous stimulation with leukaemia inhibitory factor (LIF) and small molecule inhibition of ERK1/ERK2 and GSK3beta signalling (termed 2i/LIF conditions). Hallmarks of naive pluripotency include driving Oct4 (also known as Pou5f1) transcription by its distal enhancer, retaining a pre-inactivation X chromosome state, and global reduction in DNA methylation and in H3K27me3 repressive chromatin mark deposition on developmental regulatory gene promoters. Upon withdrawal of 2i/LIF, naive mouse ES cells can drift towards a primed pluripotent state resembling that of the post-implantation epiblast. Although human ES cells share several molecular features with naive mouse ES cells, they also share a variety of epigenetic properties with primed murine epiblast stem cells (EpiSCs). These include predominant use of the proximal enhancer element to maintain OCT4 expression, pronounced tendency for X chromosome inactivation in most female human ES cells, increase in DNA methylation and prominent deposition of H3K27me3 and bivalent domain acquisition on lineage regulatory genes. The feasibility of establishing human ground state naive pluripotency in vitro with equivalent molecular and functional features to those characterized in mouse ES cells remains to be defined. Here we establish defined conditions that facilitate the derivation of genetically unmodified human naive pluripotent stem cells from already established primed human ES cells, from somatic cells through induced pluripotent stem (iPS) cell reprogramming or directly from blastocysts. The novel naive pluripotent cells validated herein retain molecular characteristics and functional properties that are highly similar to mouse naive ES cells, and distinct from conventional primed human pluripotent cells. This includes competence in the generation of cross-species chimaeric mouse embryos that underwent organogenesis following microinjection of human naive iPS cells into mouse morulas. Collectively, our findings establish new avenues for regenerative medicine, patient-specific iPS cell disease modelling and the study of early human development in vitro and in vivo.
Activation of JNKs is essential for BMP9-induced osteogenic differentiation of mesenchymal stem cells.[Pubmed:23977991]
BMB Rep. 2013 Aug;46(8):422-7.
Although BMP9 is highly capable of promoting osteogenic differentiation of mesenchymal stem cell (MSCs), the molecular mechanism involved remains to be fully elucidated. Here, we explore the possible involvement and detail role of JNKs (c-Jun N-terminal kinases) in BMP9-induced osteogenic differentiation of MSCs. It was found that BMP9 stimulated the activation of JNKs in MSCs. BMP9-induced osteogenic differentiation of MSCs was dramatically inhibited by JNKs inhibitor SP600125. Moreover, BMP9-activated Smads signaling was decreased by SP600125 treatment in MSCs. The effects of inhibitor are reproduced with adenoviruses expressing siRNA targeted JNKs. Taken together, our results revealed that JNKs was activated in BMP9-induced osteogenic differentiation of MSCs. What is most noteworthy, however, is that inhibition of JNKs activity resulted in reduction of BMP9-induced osteogenic differentiation of MSCs, implying that activation of JNKs is essential for BMP9 osteoinductive activity.
SP600125, a selective JNK inhibitor, protects ischemic renal injury via suppressing the extrinsic pathways of apoptosis.[Pubmed:17459422]
Life Sci. 2007 May 8;80(22):2067-75.
Accumulating evidence suggests that c-Jun N-terminal kinase (JNK) signaling pathway plays a critical role in renal ischemia/reperfusion injury. However, the downstream mechanism that accounts for the proapoptotic actions of JNK during renal ischemia/reperfusion has not been elucidated. We report that SP600125, a potent, cell-permeable, selective, and reversible inhibitor of c-Jun N-terminal kinase (JNK), potently decreased renal epithelial tubular cell apoptosis induced by renal ischemia/reperfusion via suppression of the extrinsic pathway. This corresponds to the decrease in JNK phosphorylation at 20 min and c-Jun phosphorylation (Ser63/73) at 3 h after renal ischemia. Additionally, SP600125 attenuated the increased expression of FasL induced by ischemia/reperfusion at 3 h. The administration of SP600125 prior to ischemia was also protective. Thus, our findings imply that SP600125 can inhibit the activation of the JNK-c-Jun-FasL pathway and protect renal tubular epithelial cells against ischemia/reperfusion-induced apoptosis. Taken together, these results indicate that targeting the JNK pathway provides a promising therapeutic approach for renal ischemia/reperfusion injury.
SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase.[Pubmed:11717429]
Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13681-6.
Jun N-terminal kinase (JNK) is a stress-activated protein kinase that can be induced by inflammatory cytokines, bacterial endotoxin, osmotic shock, UV radiation, and hypoxia. We report the identification of an anthrapyrazolone series with significant inhibition of JNK1, -2, and -3 (K(i) = 0.19 microM). SP600125 is a reversible ATP-competitive inhibitor with >20-fold selectivity vs. a range of kinases and enzymes tested. In cells, SP600125 dose dependently inhibited the phosphorylation of c-Jun, the expression of inflammatory genes COX-2, IL-2, IFN-gamma, TNF-alpha, and prevented the activation and differentiation of primary human CD4 cell cultures. In animal studies, SP600125 blocked (bacterial) lipopolysaccharide-induced expression of tumor necrosis factor-alpha and inhibited anti-CD3-induced apoptosis of CD4(+) CD8(+) thymocytes. Our study supports targeting JNK as an important strategy in inflammatory disease, apoptotic cell death, and cancer.
TAK1/JNK and p38 have opposite effects on rat hepatic stellate cells.[Pubmed:11679966]
Hepatology. 2001 Nov;34(5):953-63.
After liver injury, hepatic stellate cells (HSCs) undergo a process of activation with expression of smooth muscle alpha-actin (alpha-SMA), an increased proliferation rate, and a dramatic increase in synthesis of type I collagen. The intracellular signaling mechanisms of activation and perpetuation of the activated phenotype in HSCs are largely unknown. In this study the role of the stress-activated protein kinases, c-Jun N-terminal kinase (JNK) and p38, were evaluated in primary cultures of rat HSCs. The effect of JNK was assessed by using an adenovirus expressing a dominant negative form of transforming growth factor beta (TGF-beta)-activated kinase 1 (TAK1) (Ad5dnTAK1) and a new selective pharmacologic inhibitor SP600125. The effect of p38 was assessed with the selective pharmacologic inhibitor SB203580. These kinases were inhibited starting either in quiescent HSCs (culture day 1) or in activated HSCs (culture day 5). Although blocking TAK1/JNK and p38 decreased the expression of alpha-SMA protein in early stages of HSC activation, no effect was observed when TAK1/JNK or p38 were inhibited in activated HSCs. JNK inhibition increased and p38 inhibition decreased collagen alpha1(I) mRNA level as measured by RNase protection assays, with maximal effects observed in early stages of HSC activation. Furthermore, TAK1/JNK inhibition decreased HSC proliferation, whereas p38 inhibition led to an increased proliferation rate of HSCs, independently of its activation status. These results show novel roles for the TAK1/JNK pathway and p38 during HSC activation in culture. Despite similar activators of TAK1/JNK and p38, their functions in HSCs are distinct and opposed.


