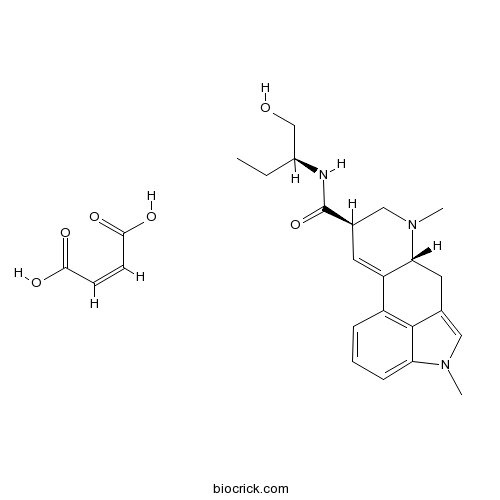
Methysergide maleate5-HT1/5-HT2 antagonist CAS# 129-49-7 |
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 129-49-7 | SDF | Download SDF |
| PubChem ID | 5281073 | Appearance | Powder |
| Formula | C25H31N3O6 | M.Wt | 469.54 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 10 mM in water with gentle warming | ||
| Chemical Name | (6aR,9R)-N-[(2S)-1-hydroxybutan-2-yl]-4,7-dimethyl-6,6a,8,9-tetrahydroindolo[4,3-fg]quinoline-9-carboxamide;(Z)-but-2-enedioic acid | ||
| SMILES | CCC(CO)NC(=O)C1CN(C2CC3=CN(C4=CC=CC(=C34)C2=C1)C)C.C(=CC(=O)O)C(=O)O | ||
| Standard InChIKey | LWYXFDXUMVEZKS-ZVFOLQIPSA-N | ||
| Standard InChI | InChI=1S/C21H27N3O2.C4H4O4/c1-4-15(12-25)22-21(26)14-8-17-16-6-5-7-18-20(16)13(10-23(18)2)9-19(17)24(3)11-14;5-3(6)1-2-4(7)8/h5-8,10,14-15,19,25H,4,9,11-12H2,1-3H3,(H,22,26);1-2H,(H,5,6)(H,7,8)/b;2-1-/t14-,15+,19-;/m1./s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mixed 5-HT1/5-HT2 receptor antagonist. |

Methysergide maleate Dilution Calculator

Methysergide maleate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1297 mL | 10.6487 mL | 21.2974 mL | 42.5949 mL | 53.2436 mL |
| 5 mM | 0.4259 mL | 2.1297 mL | 4.2595 mL | 8.519 mL | 10.6487 mL |
| 10 mM | 0.213 mL | 1.0649 mL | 2.1297 mL | 4.2595 mL | 5.3244 mL |
| 50 mM | 0.0426 mL | 0.213 mL | 0.4259 mL | 0.8519 mL | 1.0649 mL |
| 100 mM | 0.0213 mL | 0.1065 mL | 0.213 mL | 0.4259 mL | 0.5324 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Suramin hexasodium salt
Catalog No.:BCC7079
CAS No.:129-46-4
- Maohuoside A
Catalog No.:BCN5348
CAS No.:128988-55-6
- Fargesol
Catalog No.:BCN6421
CAS No.:128855-64-1
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- FRAX597
Catalog No.:BCC4172
CAS No.:1286739-19-2
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- Ospemifene
Catalog No.:BCC5557
CAS No.:128607-22-7
- SP 600125
Catalog No.:BCC2474
CAS No.:129-56-6
- Buclizine HCl
Catalog No.:BCC4516
CAS No.:129-74-8
- Rivastigmine Tartrate
Catalog No.:BCC3851
CAS No.:129101-54-8
- Evodosin A
Catalog No.:BCN7322
CAS No.:1291053-38-7
- ENMD-2076 L-(+)-Tartaric acid
Catalog No.:BCC2185
CAS No.:1291074-87-7
- CGRP 8-37 (rat)
Catalog No.:BCC5717
CAS No.:129121-73-9
- 2-(2,2-Dimethyl-1,3-dioxolan-4-yl)propane-1,2-diol
Catalog No.:BCC8475
CAS No.:129141-48-6
- Gancaonin M
Catalog No.:BCN4757
CAS No.:129145-51-3
- Biotin-HPDP
Catalog No.:BCC3583
CAS No.:129179-83-5
- Iberiotoxin
Catalog No.:BCC6932
CAS No.:129203-60-7
- (2S,3S)-(-)-Glucodistylin
Catalog No.:BCN6156
CAS No.:129212-92-6
- 15-Dihydroepioxylubimin
Catalog No.:BCN4800
CAS No.:129214-59-1
Lower extremity arterial insufficiency after long-term methysergide maleate therapy. Its evaluation with Doppler ultrasonic velocity detector.[Pubmed:464813]
Arch Surg. 1979 Aug;114(8):964-7.
Iatrogenic ergotism is the primary source of ergot intoxication. The patient whose case is reviewed had migraine headaches and received Methysergide maleate for 13 years. She had, in July 1977, severe claudication of the lower extremities. Measurements of the peripheral arterial circulation were made using the Doppler ultrasonic velocity detector. The extent of disease and subsequent reversal were documented using arteriographic examination. Initial measurements showed the patient was able to walk for one minute and 34 seconds on a treadmill (2 mph, 10% grade) before stopping because of claudication. Symptoms cleared after drug withdrawal and repeated testing produced no claudication. The calculated index (posterior tibial/arm pressure) increased from a mean of 0.22 to 0.74 during the eight-month period following discontinuance of methysergide therapy with no recurrence of migraine headaches. A review of the literature is also presented.
International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin).[Pubmed:7938165]
Pharmacol Rev. 1994 Jun;46(2):157-203.
It is evident that in the last decade or so, a vast amount of new information has become available concerning the various 5-HT receptor types and their characteristics. This derives from two main research approaches, operational pharmacology, using selective ligands (both agonists and antagonists), and, more recently, molecular biology. Although the scientific community continues to deliberate about the hierarchy of criteria for neurotransmitter receptor characterisation, there seems good agreement between the two approaches regarding 5-HT receptor classification. In addition, the information regarding transduction mechanisms and second messengers is also entirely consistent. Thus, on the basis of these essential criteria for receptor characterisation and classification, there are at least three main groups or classes of 5-HT receptor: 5-HT1, 5-HT2, and 5-HT3. Each group is not only operationally but also structurally distinct, with each receptor group having its own distinct transducing system. The more recently identified 5-HT4 receptor almost undoubtedly represents a fourth 5-HT receptor class on the basis of operational and transductional data, but this will only be definitively shown when the cDNA for the receptor has been cloned and the amino acid sequence of the protein is known. Although those 5-HT receptors that have been fully characterised and classified to date (and, hence, named with confidence) would seem to mediate the majority of the actions of 5-HT throughout the mammalian body, not all receptors for 5-HT are fully encompassed within our scheme of classification. These apparent anomalies must be recognised and need further study. They may or may not represent new groups of 5-HT receptor or subtypes of already known groups of 5-HT receptor. Even though the cDNAs for the 5-ht1E, 5-ht1F, 5-ht5, 5-ht6, and 5-ht7 receptors have been cloned and their amino acid sequence defined, more data are necessary concerning their operational and transductional characteristics before one can be confident of the suitability of their appellations. Therefore, it is important to rationalise in concert all of the available data from studies involving both operational approaches of the classical pharmacological type and those from molecular and cellular biology.(ABSTRACT TRUNCATED AT 400 WORDS)


