FRAX597PAK inhibitor,potent and ATP-competitive CAS# 1286739-19-2 |
- IPA-3
Catalog No.:BCC4978
CAS No.:42521-82-4
- PF-3758309
Catalog No.:BCC1853
CAS No.:898044-15-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1286739-19-2 | SDF | Download SDF |
| PubChem ID | 70934541 | Appearance | Powder |
| Formula | C29H28ClN7OS | M.Wt | 558.1 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : 14.29 mg/mL (25.60 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
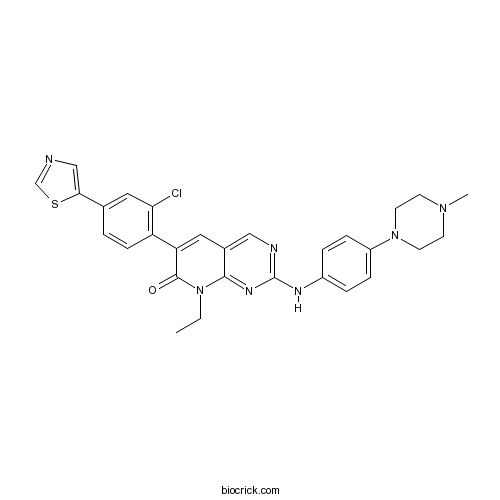
| Chemical Name | 6-[2-chloro-4-(1,3-thiazol-5-yl)phenyl]-8-ethyl-2-[4-(4-methylpiperazin-1-yl)anilino]pyrido[2,3-d]pyrimidin-7-one | ||
| SMILES | CCN1C2=NC(=NC=C2C=C(C1=O)C3=C(C=C(C=C3)C4=CN=CS4)Cl)NC5=CC=C(C=C5)N6CCN(CC6)C | ||
| Standard InChIKey | DHUJCQOUWQMVCG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H28ClN7OS/c1-3-37-27-20(14-24(28(37)38)23-9-4-19(15-25(23)30)26-17-31-18-39-26)16-32-29(34-27)33-21-5-7-22(8-6-21)36-12-10-35(2)11-13-36/h4-9,14-18H,3,10-13H2,1-2H3,(H,32,33,34) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | FRAX597 is a potent and ATP-competitive inhibitor of group I p21-activated kinases (PAKs) with IC50 values of 8 nM, 13 nM and 19 nM for PAK1, PAK2 and PAK3, respectively. | |||||
| Targets | PAK1 | PAK2 | PAK3 | |||
| IC50 | 8 nM | 13 nM | 19 nM | |||
| Cell experiment [1]: | |
| Cell lines | Nf2-null SC4 Schwann cells |
| Preparation method | Limited solubility. General tips for obtaining a higher concentration: Please warm the tube at 37 ℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months. |
| Reacting condition | 96 h |
| Applications | FRAX597 treatment significantly inhibits cellular proliferation. FRAX597-treated cells are increased in G1phase (74% versus 50% in control-treated cells) and decreased in the fraction of cells in S phase (12% versus 27% in control) and G2/M phase (11% versus 22% in control). |
| Animal experiment [1]: | |
| Animal models | NOD/SCID mice (8 weeks of age), transplanted with Nf2-/- SC4 Schwann cells into the sciatic nerve sheath. |
| Dosage form | 100 mg/kg; oral; once daily for 14 days. |
| Application | FRAX597-treatment significantly slows tumor growth rate in mice compared to control mice. Moreover, FRAX597-treated cohort exhibits prominently lower average tumor weight compared to the control cohort. |
| Other notes | Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal. |
| References: 1. Licciulli S, Maksimoska J, Zhou C et al. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas. J Biol Chem. 2013 Oct 4;288(40):29105-14. | |

FRAX597 Dilution Calculator

FRAX597 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.7918 mL | 8.959 mL | 17.9179 mL | 35.8359 mL | 44.7948 mL |
| 5 mM | 0.3584 mL | 1.7918 mL | 3.5836 mL | 7.1672 mL | 8.959 mL |
| 10 mM | 0.1792 mL | 0.8959 mL | 1.7918 mL | 3.5836 mL | 4.4795 mL |
| 50 mM | 0.0358 mL | 0.1792 mL | 0.3584 mL | 0.7167 mL | 0.8959 mL |
| 100 mM | 0.0179 mL | 0.0896 mL | 0.1792 mL | 0.3584 mL | 0.4479 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
FRAX597 is a small-molecule inhibitor of the group 1 p21-activated kinases (PAKs) with IC50 values of 8nM, 13nM and 19nM, respectively for PAK1, PAK2 and PAK3 [1].
The PAKs family includes two sub groups: 1 and 2. These kinases take participate in the growth of various types of cancers. FRAX597 is developed to be an inhibitor of group 1 PAKs from the initial hits of a high throughput screen. It is an ATP-competitive inhibitor of PAK 1-3. To group 2 PAKs, FRAX597 shows minimal inhibitory activity. The inhibition mechanism of FRAX597 is that it binds PAK by targeting the ATP binding site and competes with ATP. FRAX597 is reported to suppress cell proliferation by arresting cell cycle in G1 without impacting cell viability in Schwann cells. In vivo assay also demonstrates FRAX597 can suppress tumor growth in an orthotopic model of NF2. This effect on the cells has been proved to be mediated through the inhibition of the group I PAKs [1].
References:
[1] Silvia Licciulli, Jasna Maksimoska, Chun Zhou, Scott Troutman, Smitha Kota, Qin Liu, Sergio Duron, David Campbell, Jonathan Chernoff, Jeffery Field, Ronen Marmorstein and Joseph L. Kissil. FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits schwannomas tumorigenesis of NF2-associated. J. Biol. Chem. 2013, August.
- 1,6-O,O-Diacetylbritannilactone
Catalog No.:BCN7792
CAS No.:1286694-67-4
- PyBOP
Catalog No.:BCC2820
CAS No.:128625-52-5
- Z(2-Br)-Osu
Catalog No.:BCC2806
CAS No.:128611-93-8
- Ospemifene
Catalog No.:BCC5557
CAS No.:128607-22-7
- Pingpeimine C
Catalog No.:BCN8411
CAS No.:128585-96-6
- ML167
Catalog No.:BCC5348
CAS No.:1285702-20-6
- GSK2578215A
Catalog No.:BCC6243
CAS No.:1285515-21-0
- Romidepsin (FK228, depsipeptide)
Catalog No.:BCC3597
CAS No.:128517-07-7
- Ophiogenin-3-O-alpha-L-rhaMnopyranosyl-(1→2)-beta-D-glucopyranoside
Catalog No.:BCN1587
CAS No.:128502-94-3
- Methylophioponanone B
Catalog No.:BCN6525
CAS No.:128446-36-6
- 2-Hydroxypropyl-β-cyclodextrin
Catalog No.:BCC6757
CAS No.:128446-35-5
- Gelidoside
Catalog No.:BCN7320
CAS No.:128420-44-0
- Eucamalduside A
Catalog No.:BCN7321
CAS No.:1287220-29-4
- Kalopanaxsaponin H
Catalog No.:BCN2572
CAS No.:128730-82-5
- Mycophenolate Mofetil
Catalog No.:BCC2290
CAS No.:128794-94-5
- Fmoc-D-Asp(OtBu)-OH
Catalog No.:BCC3471
CAS No.:12883-39-3
- Fargesol
Catalog No.:BCN6421
CAS No.:128855-64-1
- Maohuoside A
Catalog No.:BCN5348
CAS No.:128988-55-6
- Suramin hexasodium salt
Catalog No.:BCC7079
CAS No.:129-46-4
- Methysergide maleate
Catalog No.:BCC5698
CAS No.:129-49-7
- SP 600125
Catalog No.:BCC2474
CAS No.:129-56-6
- Buclizine HCl
Catalog No.:BCC4516
CAS No.:129-74-8
- Rivastigmine Tartrate
Catalog No.:BCC3851
CAS No.:129101-54-8
- Evodosin A
Catalog No.:BCN7322
CAS No.:1291053-38-7
FRAX597, a PAK1 inhibitor, synergistically reduces pancreatic cancer growth when combined with gemcitabine.[Pubmed:26774265]
BMC Cancer. 2016 Jan 16;16:24.
BACKGROUND: Pancreatic ductal adenocarcinoma remains one of the most lethal of all solid tumours. Treatment options are limited and gemcitabine-based chemotherapy remains the standard of care. Although growing evidence shows that p21-activated kinase 1 (PAK1) plays a crucial role in pancreatic cancer, its role has not been fully elucidated. This study aimed to characterise the expression and functional relevance of PAK1 in pancreatic cancer. METHODS: PAK1 expression was measured in pancreatic cancer specimens by immunohistochemistry and in pancreatic cancer cell lines by western blotting. The effect of inhibition of PAK1 by either shRNA knock-down (KD), or by a selective inhibitor, FRAX597, alone or in combination with gemcitabine, on cell proliferation and migration/invasion was measured by thymidine uptake and Boyden chamber assays, respectively. The effect on tumour growth and survival was assessed in orthotopic murine models. RESULTS: PAK1 was expressed in all human pancreatic cancer samples tested, an7d was upregulated in all pancreatic cancer cell lines tested. PAK1 KD inhibited pancreatic cancer cell growth and survival, and increased sensitivity to gemcitabine treatment. AKT activity and HIF1alpha expression were also inhibited. FRAX597 inhibited pancreatic cancer cell proliferation, survival, and migration/invasion. When combined with gemcitabine, FRAX597 synergistically inhibited pancreatic cancer proliferation in vitro and inhibited tumour growth in vivo. CONCLUSIONS: These results implicate PAK1 as a regulator of pancreatic cancer cell growth and survival. Combination of a PAK1 inhibitor such as FRAX597 with cytotoxic chemotherapy deserves further study as a novel therapeutic approach to pancreatic cancer treatment.
FRAX597, a small molecule inhibitor of the p21-activated kinases, inhibits tumorigenesis of neurofibromatosis type 2 (NF2)-associated Schwannomas.[Pubmed:23960073]
J Biol Chem. 2013 Oct 4;288(40):29105-14.
The p21-activated kinases (PAKs) are immediate downstream effectors of the Rac/Cdc42 small G-proteins and implicated in promoting tumorigenesis in various types of cancer including breast and lung carcinomas. Recent studies have established a requirement for the PAKs in the pathogenesis of Neurofibromatosis type 2 (NF2), a dominantly inherited cancer disorder caused by mutations at the NF2 gene locus. Merlin, the protein product of the NF2 gene, has been shown to negatively regulate signaling through the PAKs and the tumor suppressive functions of Merlin are mediated, at least in part, through inhibition of the PAKs. Knockdown of PAK1 and PAK2 expression, through RNAi-based approaches, impairs the proliferation of NF2-null schwannoma cells in culture and inhibits their ability to form tumors in vivo. These data implicate the PAKs as potential therapeutic targets. High-throughput screening of a library of small molecules combined with a structure-activity relationship approach resulted in the identification of FRAX597, a small-molecule pyridopyrimidinone, as a potent inhibitor of the group I PAKs. Crystallographic characterization of the FRAX597/PAK1 complex identifies a phenyl ring that traverses the gatekeeper residue and positions the thiazole in the back cavity of the ATP binding site, a site rarely targeted by kinase inhibitors. FRAX597 inhibits the proliferation of NF2-deficient schwannoma cells in culture and displayed potent anti-tumor activity in vivo, impairing schwannoma development in an orthotopic model of NF2. These studies identify a novel class of orally available ATP-competitive Group I PAK inhibitors with significant potential for the treatment of NF2 and other cancers.


