CC-401 hydrochlorideJNK inhibitor CAS# 1438391-30-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1438391-30-0 | SDF | Download SDF |
| PubChem ID | 66576998 | Appearance | Powder |
| Formula | C22H25ClN6O | M.Wt | 424.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CC401 HCl | ||
| Solubility | DMSO : 100 mg/mL (235.33 mM; Need ultrasonic) | ||
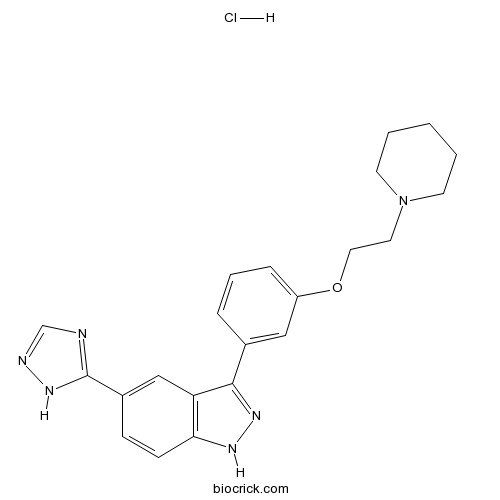
| Chemical Name | 3-[3-(2-piperidin-1-ylethoxy)phenyl]-5-(1H-1,2,4-triazol-5-yl)-1H-indazole;hydrochloride | ||
| SMILES | C1CCN(CC1)CCOC2=CC=CC(=C2)C3=NNC4=C3C=C(C=C4)C5=NC=NN5.Cl | ||
| Standard InChIKey | OIBVXKYKWOUGAO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H24N6O.ClH/c1-2-9-28(10-3-1)11-12-29-18-6-4-5-16(13-18)21-19-14-17(22-23-15-24-27-22)7-8-20(19)25-26-21;/h4-8,13-15H,1-3,9-12H2,(H,25,26)(H,23,24,27);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | CC-401 hydrochloride is a potent inhibitor of all three forms of JNK with Ki of 25 to 50 nM.In Vitro:CC-401 has at least 40-fold selectivity for JNK compared with other related kinases, including p38, extracellular signal-regulated kinase (ERK), inhibitor of κB kinase (IKK2), protein kinase C, Lck, zeta-associated protein of 70 kDa (ZAP70). In cell-based assays, 1 to 5 μM CC-401 provides specific JNK inhibition. CC-401, a small molecule that is a specific inhibitor of all three JNK isoforms. CC-401 competitively binds the ATP binding site in JNK, resulting in inhibition of the phosphorylation of the N-terminal activation domain of the transcription factor c-Jun. The specificity of this inhibitor is tested in vitro using osmotic stress of the HK-2 human tubular epithelial cell line. CC-401 inhibits sorbitol-induced phosphorylation of c-Jun in a dosage-dependent manner. However, CC-401 does not prevent sorbitol-induced phosphorylation of JNK, p38, or ERK[1].In Vivo:The staining of p-JNK is moderately induced in bevazicumab and Oxaliplatin treatments as compared to control, and in the CC-401-treated samples p-cJun content is significantly lower, consistent with effective JNK inhibition. DNA damage is modestly elevated in combined treatments with CC-401[2]. CC-401 treatment from days 7 to 24 slows the progression of proteinuria, which is significantly reduced compared to the no-treatment and vehicle groups at days 14 and 21. However, there is still an increase in the degree of proteinuria at day 21 in CC-401-treated rats compared to proteinuria at day 5. The vehicle and no-treatment groups developed renal impairment at day 24 as shown by an increase in serum creatinine. This is prevented by CC-401 treatment[3]. References: | |||||

CC-401 hydrochloride Dilution Calculator

CC-401 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3533 mL | 11.7666 mL | 23.5333 mL | 47.0666 mL | 58.8332 mL |
| 5 mM | 0.4707 mL | 2.3533 mL | 4.7067 mL | 9.4133 mL | 11.7666 mL |
| 10 mM | 0.2353 mL | 1.1767 mL | 2.3533 mL | 4.7067 mL | 5.8833 mL |
| 50 mM | 0.0471 mL | 0.2353 mL | 0.4707 mL | 0.9413 mL | 1.1767 mL |
| 100 mM | 0.0235 mL | 0.1177 mL | 0.2353 mL | 0.4707 mL | 0.5883 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CC-401 is a second generation ATP-competitive anthrapyrazolone c-Jun N terminal kinase (JNK) inhibitor with potential antineoplastic activity. Based on the chemistry of SP600125, another anthrapyrazolone inhibitor of JNK, CC-401 competitively binds the ATP binding site of JNK, resulting in inhibition of the phosphorylation of the N-terminal activation domain of transcription factor c-Jun; decreased transcription activity of c-Jun; and a variety of cellular effects including decreased cellular proliferation.
- 2,24-Dihydroxyursolic acid
Catalog No.:BCN6244
CAS No.:143839-02-5
- Fmoc-Trp(Boc)-OH
Catalog No.:BCC3558
CAS No.:143824-78-6
- 22-Dehydroclerosterol glucoside
Catalog No.:BCN6243
CAS No.:143815-99-0
- 13-Epimanool
Catalog No.:BCN4862
CAS No.:1438-62-6
- SB 200646 hydrochloride
Catalog No.:BCC5751
CAS No.:143797-62-0
- 3-O-Coumaroylasiatic acid
Catalog No.:BCN7132
CAS No.:143773-52-8
- (RS)-Abscisic acid
Catalog No.:BCN8353
CAS No.:14375-45-2
- PACAP 6-38
Catalog No.:BCC7611
CAS No.:143748-18-9
- Pyrazine-2-carbaldehyde
Catalog No.:BCN2565
CAS No.:5780-66-5
- Kaempferol 3,4,7-triacetate
Catalog No.:BCN6242
CAS No.:143724-69-0
- Soyasaponin Bd
Catalog No.:BCN2465
CAS No.:135272-91-2
- CE3F4
Catalog No.:BCC5605
CAS No.:143703-25-7
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
- M40
Catalog No.:BCC7686
CAS No.:143896-17-7
- CB-839
Catalog No.:BCC5493
CAS No.:1439399-58-2
- Jaceidin triacetate
Catalog No.:BCN6245
CAS No.:14397-69-4
- CTX0294885
Catalog No.:BCC6396
CAS No.:1439934-41-4
- Sodium barbital
Catalog No.:BCN2160
CAS No.:144-02-5
- Sodium bicarbonate
Catalog No.:BCC7584
CAS No.:144-55-8
- Oxalic acid
Catalog No.:BCN8515
CAS No.:144-62-7
- Zeaxanthin
Catalog No.:BCN2380
CAS No.:144-68-3
- Sulfathiazole sodium
Catalog No.:BCC5207
CAS No.:144-74-1
- Sulfamethizole
Catalog No.:BCC4856
CAS No.:144-82-1
- Sulfapyridine
Catalog No.:BCC4729
CAS No.:144-83-2
Identification of pyrolysis products of the new psychoactive substance 2-amino-1-(4-bromo-2,5-dimethoxyphenyl)ethanone hydrochloride (bk-2C-B) and its iodo analogue bk-2C-I.[Pubmed:28371351]
Drug Test Anal. 2018 Jan;10(1):229-236.
2-Amino-1-(4-bromo-2,5-dimethoxyphenyl)ethanone hydrochloride (bk-2C-B) has recently emerged as a new psychoactive substance (NPS). It is most commonly consumed orally, although there are indications that it might also be ingested by inhalation or 'smoking'. Information about the stability of bk-2C-B when exposed to heat is unavailable and the potential for pyrolytic degradation and formation of unknown substances available for inhalation prompted an investigation using a simulated 'meth pipe' scenario. Twelve products following pyrolysis of bk-2C-B were detected and verified by organic synthesis of the corresponding standards. In addition, 2-amino-1-(4-iodo-2,5-dimethoxyphenyl)ethanone hydrochloride (bk-2C-I) was characterized for the first time and subjected to pyrolysis as well. Similar products were formed, which indicated that the replacement of the bromo with the iodo substituent did not affect the pyrolysis pattern under the conditions used. Two additional products were detected in the bk-2C-I pyrolates, namely 1-(2,5-dimethoxyphenyl)-ethanone and 1-iodo-4-ethenyl-5-methoxyphenol. The potential ingestion of pyrolysis products with unknown toxicity adds an element of concern. Copyright (c) 2017 John Wiley & Sons, Ltd.
Compatibility and Stability of Rolapitant Injectable Emulsion Admixed with Intravenous Palonosetron Hydrochloride.[Pubmed:28346200]
Int J Pharm Compd. 2017 Jan-Feb;21(1):76-82.
Neurokinin-1 receptor antagonist, 5-hydroxytryptamine-3 RA, and dexamethasone combination therapy is standard of care for the prevention of chemotherapy-induced nausea and vomiting. Herein we describe the physical and chemical stability of rolapitant injectable emulsion 166.5 mg in 92.5 mL (185 mg hydrochloride salt) admixed with palonosetron injection 0.25 mg in 5 mL (0.28 mg hydrochloride salt). Admixtures were prepared and stored in two types of container closures (110-mL Crystal Zenith plastic and glass bottles) and four types of intravenous administration sets (or intravenous tubing sets). Assessment of the physical and chemical stability was conducted on the admixtures in the ready-to-use container closure systems as supplied by the manufacturer, stored at room temperature (20 degrees C to 25 degrees C under fluorescent light), and evaluated at 0, 1, and 6 hours; 1 and 2 days; and under refrigeration (2 degrees C to 8 degrees C protected from light) after 1, 3, and 7 days. For admixtures in intravenous tubing sets, the assessment of physicochemical stability was performed after 0 and 7 hours of storage at 20 degrees C to 25 degrees C initially, and then after 20 hours (total 27 hours) at 2 degrees C to 8 degrees C protected from light. Physical stability was assessed by visual examination of the container contents under normal room light, and measuring turbidity and particulate matter. Chemical stability was assessed by measuring the pH of the admixture and determining drug concentrations and impurity levels with high-performance liquid chromatographic analysis. The results indicated that all samples were physically compatible throughout the duration of the study. The pH, turbidity, and particulate matter of the admixture stayed within narrow and acceptable ranges. Rolapitant admixed with palonosetron was chemically stable when admixed in glass and Crystal Zenith bottles for at least 48 hours at room temperature and for 7 days under refrigeration, as well as in the four selected intravenous tubing sets for 7 hours at 20 degrees C to 25 degrees C and then for 20 hours at 2 degrees C to 8 degrees C. No loss of potency of any admixed components occurred in the samples stored at the two temperature ranges and time period studied.
[The Discovery, Research and Development of Etelcalcetide Hydrochloride, the World 1st Intravenous Calcimimetics.][Pubmed:28336830]
Clin Calcium. 2017;27(4):537-545.
Etelcalcetide hydrochloride is the first intravenous calcimimetics agent for secondary hyperparathyroidism (SHPT). Etelcalcetide hydrochloride is to be administered through dialysis circuit by physician or medical staff upon completion of dialysis and such administration is expected to reduce the burden of medication in patients. From the nonclinical study results, etelcalcetide functions as an allosteric activator of calcium-sensing receptor(CaSR). Etelcalcetide suppressed PTH secretion both in vitro and in vivo. In a rat model of chronic renal insufficiency, etelcalcetide suppressed SHPT disorders, such as parathyroid gland hypertrophy, bone disorder, and ectopic calcification. In conclusion, etelcalcetide hydrochloride is expected to exhibit therapeutic effect against each SHPT condition by decreasing blood PTH concentrations via CaSR-agonist activity in the clinical situation.
Study on the interaction of 6-(2-morpholin-4-yl-ethyl)-6H-indolo [2,3-b]quinoxaline hydrochloride with human serum albumin by fluorescence spectroscopy.[Pubmed:28355158]
Methods Appl Fluoresc. 2016 Sep 14;4(3):034012.
Under physiological conditions, in vitro interaction between the bio-active substance 6-(2-morpholin-4-yl-ethyl)-6H-indolo[2,3-b]quinoxaline hydrochloride (MIQ) and human serum albumin (HSA) was investigated at an excitation wavelength 260 nm and at different temperatures (298 K, 308 K and 313 K) by fluorescence emission spectroscopy. From spectral analysis, MIQ showed a strong ability to quench the intrinsic fluorescence of HSA through a static quenching procedure. The binding constant is estimated asK A = 2.55 x 10(-4) l . mol(-1) at 298 K. Based on the thermodynamic parameters evaluated from the van 't Hoff equation, the enthalpy change (DeltaH degrees ) and entropy change (DeltaS degrees ) were derived to be negative values. A value of 2.37 nm for the average distance r between MIQ (acceptor) and tryptophan residues of HSA (donor) was derived from the fluorescence resonance energy transfer. UV/vis absorption spectra were used to confirm the quenching mechanism.


