Oxalic acidCAS# 144-62-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144-62-7 | SDF | Download SDF |
| PubChem ID | 971 | Appearance | White powder |
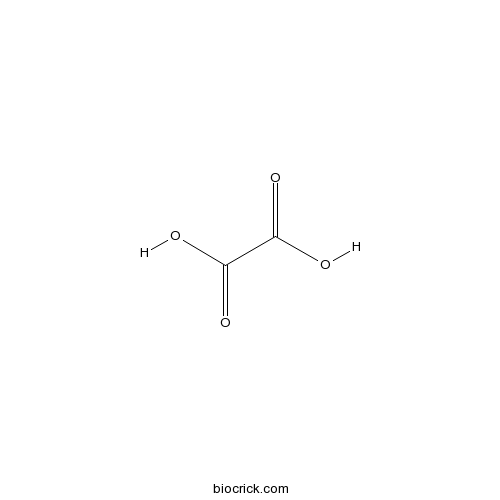
| Formula | C2H2O4 | M.Wt | 90.03 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | oxalic acid | ||
| SMILES | OC(=O)C(O)=O | ||
| Standard InChIKey | MUBZPKHOEPUJKR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C2H2O4/c3-1(4)2(5)6/h(H,3,4)(H,5,6) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Oxalic acid Dilution Calculator

Oxalic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 11.1074 mL | 55.537 mL | 111.0741 mL | 222.1482 mL | 277.6852 mL |
| 5 mM | 2.2215 mL | 11.1074 mL | 22.2148 mL | 44.4296 mL | 55.537 mL |
| 10 mM | 1.1107 mL | 5.5537 mL | 11.1074 mL | 22.2148 mL | 27.7685 mL |
| 50 mM | 0.2221 mL | 1.1107 mL | 2.2215 mL | 4.443 mL | 5.5537 mL |
| 100 mM | 0.1111 mL | 0.5554 mL | 1.1107 mL | 2.2215 mL | 2.7769 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Sodium bicarbonate
Catalog No.:BCC7584
CAS No.:144-55-8
- Sodium barbital
Catalog No.:BCN2160
CAS No.:144-02-5
- CTX0294885
Catalog No.:BCC6396
CAS No.:1439934-41-4
- Jaceidin triacetate
Catalog No.:BCN6245
CAS No.:14397-69-4
- CB-839
Catalog No.:BCC5493
CAS No.:1439399-58-2
- M40
Catalog No.:BCC7686
CAS No.:143896-17-7
- Elacridar hydrochloride
Catalog No.:BCC1547
CAS No.:143851-98-3
- CC-401 hydrochloride
Catalog No.:BCC1458
CAS No.:1438391-30-0
- 2,24-Dihydroxyursolic acid
Catalog No.:BCN6244
CAS No.:143839-02-5
- Fmoc-Trp(Boc)-OH
Catalog No.:BCC3558
CAS No.:143824-78-6
- 22-Dehydroclerosterol glucoside
Catalog No.:BCN6243
CAS No.:143815-99-0
- 13-Epimanool
Catalog No.:BCN4862
CAS No.:1438-62-6
- Zeaxanthin
Catalog No.:BCN2380
CAS No.:144-68-3
- Sulfathiazole sodium
Catalog No.:BCC5207
CAS No.:144-74-1
- Sulfamethizole
Catalog No.:BCC4856
CAS No.:144-82-1
- Sulfapyridine
Catalog No.:BCC4729
CAS No.:144-83-2
- Sodium Nitroprusside
Catalog No.:BCC4844
CAS No.:14402-89-2
- BRD73954
Catalog No.:BCC5652
CAS No.:1440209-96-0
- Piclamilast
Catalog No.:BCC6215
CAS No.:144035-83-6
- 6-O-Syringoylajugol
Catalog No.:BCN6246
CAS No.:144049-72-9
- Febuxostat
Catalog No.:BCC2556
CAS No.:144060-53-7
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
Electrodegradation of naphthalenic amines: Influence of the relative position of the substituent groups, anode material and electrolyte on the degradation products and kinetics.[Pubmed:29705634]
Chemosphere. 2018 Aug;205:433-442.
The electrodegradation of the 4-aminonaphthalene-1-sulfonic acid (4AN1S), 5-aminonaphthalene-2-sulfonic acid (5AN2S) and 8-aminonaphthalene-2-sulfonic acid (8AN2S) was studied, using two electrode materials as anode, BDD and Ti/Pt/PbO2, and two different electrolytes, sodium sulfate and sodium chloride. The highest COD removal rates were obtained at BDD: for 5AN2S and 8AN2S results were similar in both electrolytes; for 4AN1S, results were better in sodium chloride. The lowest COD removal rates were obtained at the system Ti/Pt/PbO2-sodium sulfate, for all the studied amines. The dissolved organic carbon (DOC) removal was much higher at BDD for all the amines, in sulfate for 5AN2S and 8AN2S and in chloride for 4AN1S. Nitrogen removal was always almost irrelevant in sulfate medium but higher than 60%, after 6-h assays, in chloride. The highest combustion efficiencies were attained at the system BDD-sodium sulfate and were: 4AN1S-75%; 5AN2S-84%; 8AN2S-74%. HPLC results show that total degradation of the studied aminonaphthalene sulfonates is attained at both anode materials, utilizing any of the electrolytes, with a first order kinetics. However, kinetic constants obtained with the variation of the amines concentration in time are 10-40 times higher in chloride, being slightly higher at Ti/Pt/PbO2 than at BDD. Regarding the presence of carboxylic acids during the degradation assays, it was observed that the electrolysis of the amines 5AN2S and 8AN2S always lead to higher amounts of Oxalic acid and lower quantities of acetic acid than the electrolysis of the amine 4AN1S.
Hyperoside protects human kidney2 cells against oxidative damage induced by oxalic acid.[Pubmed:29750296]
Mol Med Rep. 2018 Jul;18(1):486-494.
The majority of renal calculi (kidney stones) are calcium stones. Oxidative damage to renal tubular epithelial cells induced by reactive oxygen species (ROS) is the predominant cause of calcium oxalate stone formation. Hyperoside (Hyp) is a flavonol glycoside extracted from medicinal plants and appears to exhibit potent antioxidant activity in various cells. The aim of the present study was to investigate the protective effect of Hyp on renal cells exposed to oxidative stress simulated by Oxalic acid (OA), and to determine whether the underlying mechanism involves the nuclear factor E2related factor2 (Nrf2)antioxidative response element signaling pathway. The study determined the indicators of high oxidative stress, including ROS and hydrogen peroxide (H2O2) in human kidney2 cells and the results demonstrated that the levels of ROS, as evaluated by flow cytometry, and H2O2 were significantly increased following treatment with OA (5 mmol/l) for 24 h (OA group), compared with those in the untreated control group. The increased activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in these cells explained this observation, as it is a major source of ROS. The results demonstrated that, in the OA group, the adhesion of calcium oxalate crystals and lactate dehydrogenase (LDH) were significantly increased, and MTT assay demonstrated that cell viability was inhibited, compared with the control, which indicated that severe injury of cells was induced by OA. However, when the cells were pretreated with Hyp prior to treatment with OA (drug group), the levels of ROS and H2O2, and the activities of NADPH oxidase and LD were increased, and the adhesion of calcium oxalate crystals to cells was reduced, compared with the OA group. Western blot analysis and reverse transcriptionquantitative polymerase chain reaction demonstrated that the protein and mRNA expression levels of Nrf2, heme oxygenase1 (HO1) and NAD(P)H: quinineoxidoreductase 1 (NQO1) in the Hyp groups were significantly increased, compared with those in the OA group, with the exception of Nrf2 mRNA. These results suggested that Hyp had a marked protective effect on renal cells against the oxidative damage and cytotoxicity simulated by OA. This is the first report, to the best of our knowledge, demonstrating that the ability of Hyp to enhance the endogenous functions of antioxidation and detoxification in cells may involve the Nrf2/HO1/NQO1 pathway.
Simultaneous application of oxalic acid and dithionite for enhanced extraction of arsenic bound to amorphous and crystalline iron oxides.[Pubmed:29729603]
J Hazard Mater. 2018 Jul 15;354:91-98.
To extract As bound to amorphous and crystalline iron oxides, this study proposed simultaneous application of Oxalic acid and dithionite, which was observed to induce synergistic effect and accomplish effective extraction of As bound to both iron oxides. However, the formation of arsenic sulfide decreased overall removal of As because the insoluble precipitate form of As remained as a residual fraction of As in soil. Therefore, stepwise addition of dithionite in the simultaneous application was applied to minimize the formation of secondary minerals and maximize the As extraction. As a result, 74% of As bound to amorphous iron oxides and 65% of As bound to crystalline iron oxides were removed. More importantly, the stepwise application of Oxalic acid and dithionite was effective to reduce the bioaccessible concentration of As in the treated soil. Therefore, the proposed application could reduce the potential risk of contaminated soil to human health by extraction-based remedial action.
Biodegradation of Lignin Monomers Vanillic, p-Coumaric, and Syringic Acid by the Bacterial Strain, Sphingobacterium sp. HY-H.[Pubmed:29750329]
Curr Microbiol. 2018 Sep;75(9):1156-1164.
Many bacterial strains have been demonstrated to biodegrade lignin for contaminant removal or resource regeneration. The goal of this study was to investigate the biodegradation amount and associated pathways of three lignin monomers, vanillic, p-coumaric, and syringic acid by strain Sphingobacterium sp. HY-H. Vanillic, p-coumaric, and syringic acid degradation with strain HY-H was estimated as 88.71, 76.67, and 72.78%, respectively, after 96 h. Correspondingly, the same three monomers were associated with a COD removal efficiency of 87.30, 55.17, and 67.23%, and a TOC removal efficiency of 82.14, 61.03, and 43.86%. The results of GC-MS, HPLC, FTIR, and enzyme activities show that guaiacol and o-dihydroxybenzene are key intermediate metabolites of the vanillic acid and syringic acid degradation. p-Hydroxybenzoic acid is an important intermediate metabolite for p-coumaric and syringic acid degradation. LiP and MnP play an important role in the degradation of lignin monomers and their intermediate metabolites. One possible pathway is that strain HY-H degrades lignin monomers into guaiacol (through decarboxylic and demethoxy reaction) or p-hydroxybenzoic acid (through side-chain oxidation); then guaiacol demethylates to o-dihydroxybenzene. The p-hydroxybenzoic acid and o-dihydroxybenzene are futher through ring cleavage reaction to form small molecule acids (butyric, valproic, Oxalic acid, and propionic acid) and alcohols (ethanol and ethanediol), then these acids and alcohols are finally decomposed into CO2 and H2O through the tricarboxylic acid cycle. If properly optimized and controlled, the strain HY-H may play a role in breaking down lignin-related compounds for biofuel and chemical production.
Conservation of Monuments by a Three-Layered Compatible Treatment of TEOS-Nano-Calcium Oxalate Consolidant and TEOS-PDMS-TiO(2) Hydrophobic/Photoactive Hybrid Nanomaterials.[Pubmed:29702571]
Materials (Basel). 2018 Apr 27;11(5). pii: ma11050684.
In the conservation of monuments, research on innovative nanocomposites with strengthening, hydrophobic and self-cleaning properties have attracted the interest of the scientific community and promising results have been obtained as a result. In this study, stemming from the need for the compatibility of treatments in terms of nanocomposite/substrate, a three-layered compatible treatment providing strengthening, hydrophobic, and self-cleaning properties is proposed. This conservation approach was implemented treating lithotypes and mortars of different porosity and petrographic characteristics with a three-layered treatment comprising: (a) a consolidant, tetraethoxysilane (TEOS)-nano-Calcium Oxalate; (b) a hydrophobic layer of TEOS-polydimethylsiloxane (PDMS); and (c) a self-cleaning layer of TiO(2) nanoparticles from titanium tetra-isopropoxide with Oxalic acid as hole-scavenger. After the three-layered treatment, the surface hydrophobicity was improved due to PDMS and nano-TiO(2) in the interface substrate/atmosphere, as proven by the homogeneity and the Si(-)O(-)Ti hetero-linkages of the blend protective/self-cleaning layers observed by Scanning Electron Microscope (SEM), Transmission Electron Microscope (TEM) and Fourier-Transform Infrared Spectroscopy (FTIR). The aesthetic, microstructural, mechanical and permeabile compatibility of the majority of treated substrates ranged within acceptability limits. The improved photocatalytic activity, as proven by the total discoloration of methylene blue in the majority of cases, was attributed to the anchorage of TiO(2), through the Si(-)O(-)Ti bonds to SiO(2), in the interface with the atmosphere, thus enhancing photoactivation.


