Febuxostatnon-purine selective inhibitor of xanthine oxidase CAS# 144060-53-7 |
- chroman 1
Catalog No.:BCC1480
CAS No.:1273579-40-0
- RKI-1447
Catalog No.:BCC1903
CAS No.:1342278-01-6
- Y-27632
Catalog No.:BCC4301
CAS No.:146986-50-7
- Y-27632 dihydrochloride
Catalog No.:BCC1273
CAS No.:129830-38-2
- GSK429286A
Catalog No.:BCC2532
CAS No.:864082-47-3
- SLx-2119
Catalog No.:BCC1954
CAS No.:911417-87-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144060-53-7 | SDF | Download SDF |
| PubChem ID | 134018 | Appearance | Powder |
| Formula | C16H16N2O3S | M.Wt | 316.37 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | TEI 6720, TMX 67 | ||
| Solubility | DMSO : 50 mg/mL (158.04 mM; Need ultrasonic) | ||
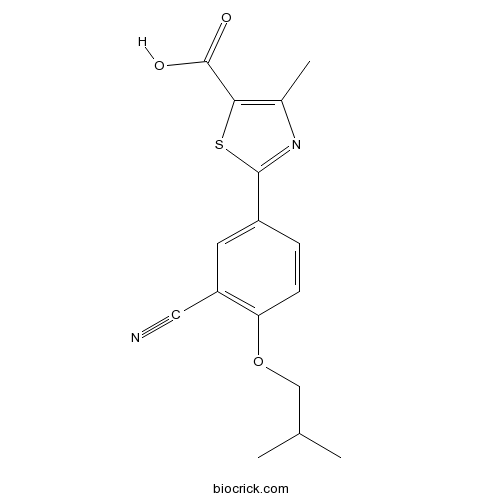
| Chemical Name | 2-[3-cyano-4-(2-methylpropoxy)phenyl]-4-methyl-1,3-thiazole-5-carboxylic acid | ||
| SMILES | CC1=C(SC(=N1)C2=CC(=C(C=C2)OCC(C)C)C#N)C(=O)O | ||
| Standard InChIKey | BQSJTQLCZDPROO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C16H16N2O3S/c1-9(2)8-21-13-5-4-11(6-12(13)7-17)15-18-10(3)14(22-15)16(19)20/h4-6,9H,8H2,1-3H3,(H,19,20) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Non-purine inhibitor of xanthine oxidase (Ki = 1.2 nM). Binds to a channel leading to the enzyme active site; obstructs substrate binding. |

Febuxostat Dilution Calculator

Febuxostat Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.1609 mL | 15.8043 mL | 31.6086 mL | 63.2171 mL | 79.0214 mL |
| 5 mM | 0.6322 mL | 3.1609 mL | 6.3217 mL | 12.6434 mL | 15.8043 mL |
| 10 mM | 0.3161 mL | 1.5804 mL | 3.1609 mL | 6.3217 mL | 7.9021 mL |
| 50 mM | 0.0632 mL | 0.3161 mL | 0.6322 mL | 1.2643 mL | 1.5804 mL |
| 100 mM | 0.0316 mL | 0.158 mL | 0.3161 mL | 0.6322 mL | 0.7902 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: Febuxostat displayed potent mixed-type inhibition of the activity of xanthine oxidase (XO), with Ki value of 0.6 nM [1]. Febuxostat was also reported to be 1000-fold (IC50=1.8 nM) more potent than allopurinol (IC50= 2.9 μM) at inhibiting XO-dependent uric acid formation [2].
Xanthine oxidase is a critical source of reactive oxygen species which contribute to vascular inflammation. Febuxostat is a non-purine selective inhibitor of xanthine oxidase. It works by non-competitively blocking the molybdenum pterin center which is the active site on xanthine oxidase. Xanthine oxidase is needed to successively oxidize both hypoxanthine and xanthine to uric acid. Hence, febuxostat inhibits xanthine oxidase, therefore reducing production of uric acid. Febuxostat inhibits both oxidized as well as reduced form of xanthine oxidase because of which febuxostat cannot be easily displaced from the molybdenum pterin site.
In vitro: In a previous study, the authors investigated the effects of febuxostat on several enzymes in purine and pyrimidine metabolism and characterized the mechanism of febuxostat inhibition of XO activity. Results showed that Febuxostat displayed potent mixed-type inhibition of the activity of purified bovine milk XO, indicating inhibition of both the oxidized and reduced forms of XO. These results demonstrate that febuxostat is a potent non-purine, selective inhibitor of XO, and could be useful for the treatment of hyperuricemia and gout. [1].
In vivo: A study evaluated whether febuxostat (Fx) could alleviate the features of metabolic syndrome as well as the renal hemodynamic alterations and afferent arteriolopathy induced by a high-fructose diet in rats. Compared with fructose, fructose+Fx rats showed significantly lowered blood pressure, UA, triglycerides, and insulin. Moreover, fructose+Fx rats had significantly reduced glomerular pressure, renal vasoconstriction, and afferent arteriolar area relative to fructose rats. These results provide further evidence for a pathogenic role of hyperuricemia in fructose-mediated metabolic syndrome [3].
Clinical trial: Febuxostat (INN; trade names Adenuric in Europe and New Zealand and Uloric in the US) is drug that is indicated for use in the treatment of chronic gout and hyperuricemia. It acts as an inhibitor of xanthine oxidase, thus lowering urate concentrations in the body. Febuxostat received marketing approval by the European Medicines Agency for Menarini on April 21, 2008 and was approved by the U.S. Food and Drug Administration for Takeda on February 16, 2009.
Reference:
[1] Yasuhiro Takano, Kumiko Hase-Aoki, Hideki Horiuchi, Lin Zhao, Yoshinori Kasahara, Shiro Kondo, Michael A. Becker. Selectivity of febuxostat, a novel non-purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sciences 76 (2005) 1835–1847
[2] Umair Z. Malik, Nicholas J. Hundley, Guillermo Romero, Rafael Radi, Bruce A. Freeman, Margaret M. Tarpey, Eric E. Kelley. Febuxostat inhibition of endothelial-bound XO: Implications for targeting vascular ROS production. Free Radical Biol Med 51(2011) 179-184
[3] Laura G. Sa´nchez-Lozada,Edilia Tapia, Pablo Bautista-Garcı´a, Virgilia Soto, Carmen A´ vila-Casado, Iliana P. Vega-Campos, Takahiko Nakagawa, Lin Zhao, Martha Franco, and Richard J. Johnson. Effects of febuxostat on metabolic and renal alterations in rats with fructose-induced metabolic syndrome. Am J Physiol Renal Physiol 294: F710–F718, 2008.
- 6-O-Syringoylajugol
Catalog No.:BCN6246
CAS No.:144049-72-9
- Piclamilast
Catalog No.:BCC6215
CAS No.:144035-83-6
- BRD73954
Catalog No.:BCC5652
CAS No.:1440209-96-0
- Sodium Nitroprusside
Catalog No.:BCC4844
CAS No.:14402-89-2
- Sulfapyridine
Catalog No.:BCC4729
CAS No.:144-83-2
- Sulfamethizole
Catalog No.:BCC4856
CAS No.:144-82-1
- Sulfathiazole sodium
Catalog No.:BCC5207
CAS No.:144-74-1
- Zeaxanthin
Catalog No.:BCN2380
CAS No.:144-68-3
- Oxalic acid
Catalog No.:BCN8515
CAS No.:144-62-7
- Sodium bicarbonate
Catalog No.:BCC7584
CAS No.:144-55-8
- Sodium barbital
Catalog No.:BCN2160
CAS No.:144-02-5
- CTX0294885
Catalog No.:BCC6396
CAS No.:1439934-41-4
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Fmoc-Glu-OAll
Catalog No.:BCC3490
CAS No.:144120-54-7
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Indoxacarb
Catalog No.:BCN2263
CAS No.:144171-61-9
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- YYA-021
Catalog No.:BCC5346
CAS No.:144217-65-2
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
Ameliorative effects of sildenafil and/or febuxostat on doxorubicin-induced nephrotoxicity in rats.[Pubmed:28257823]
Eur J Pharmacol. 2017 Jun 15;805:118-124.
Sildenafil and Febuxostat protect against doxorubicin-induced nephrotoxicity; however the exact mechanism remains to be elucidated. The effect of sildenafil and Febuxostat on doxorubicin-induced nephrotoxicity in rats was studied. Male rats were subdivided into nine groups. The 1st group served as normal control, the 2nd group received dimethylsulfoxide 50% (DMSO), the 3rd group received doxorubicin (3.5mg/kg, i.p.), twice weekly for 3 weeks. The next 3 groups received sildenafil (5mg/kg; p.o.), Febuxostat (10mg/kg; p.o.) and their combination, respectively daily for 21 days. The last 3 groups received doxorubicin in combination with sildenafil, Febuxostat or their combination. Nephrotoxicity was evaluated histopathologically by light microscopy and biochemically through measuring the following parameters, Kidney function biomarkers [serum levels of urea, creatinine and uric acid], oxidative stress biomarkers [kidney contents of glutathione reduced (GSH) and malondialdehyde (MDA)], The apoptotic marker namely; caspase-3 in kidney tissue and the inflammatory mediator tumor necrosis factor alpha (TNF-alpha). doxorubicin-induced a significant elevation in nephrotoxicity markers, expression of caspase-3 and caused induction of inflammation and oxidative stress. Histological changes in the kidney was tubular necrosis. Sildenafil and/or Febuxostat administration with doxorubicin caused a significant decrease in nephrotoxicity markers and inflammatory mediators, restoration of normal values of oxidative stress biomarkers and hampering the expression of renal caspase-3. They also ameliorate histological changes induced by doxorubicin. sildenafil and Febuxostat are promising protective agents against doxorubicin-nephrotoxicity through improving biochemical, inflammatory, histopathological and immunohistochemical alterations induced by doxorubicin.
Risk of Febuxostat-Associated Myopathy in Patients with CKD.[Pubmed:28302902]
Clin J Am Soc Nephrol. 2017 May 8;12(5):744-750.
BACKGROUND AND OBJECTIVES: Febuxostat, a nonpurine xanthine oxidase inhibitor, is widely used to treat hyperuricemia. Although Febuxostat-associated rhabdomyolysis was reported in some patients with CKD, the association between CKD and Febuxostat-associated myopathy remains uncertain. DESIGN, SETTING, PARTICIPANTS, & MEASUREMENTS: Our retrospective cohort study included 1332 patients using Febuxostat in Taipei Medical University-Wanfang Hospital from February of 2014 to January of 2016. The primary predictor was time-averaged eGFR as calculated by the equation proposed by the 2009 Chronic Kidney Disease Epidemiology Collaboration. The outcome was Febuxostat-associated myopathy defined as elevated creatine kinase levels during Febuxostat use that were not attributed to other muscular injuries. RESULTS: The median duration of Febuxostat use was 224 days (25th, 75th percentiles: 86, 441.5 days). Of 1332 study participants, 1222 (91.7%) had CKD; the median eGFR was 20.8 ml/min per 1.73 m(2) (25th, 75th percentiles: 9.0, 35.4 ml/min per 1.73 m(2)). Forty-one of the participants had Febuxostat-associated myopathy (3.2%). All patients with myopathy had CKD, and the incident rate was 0.013 (95% confidence interval, 0.01 to 0.02) events per 100 patient-days in patients with CKD. Of 41 patients with myopathy, 37 had myositis, and four had rhabdomyolysis. Myopathy resolved in 17 patients who withdrew from treatment and eight patients who continued Febuxostat treatment. Among the evaluated predictors, multivariate analysis showed that only the lowest eGFR tertile was significantly associated with myopathy in Febuxostat users. The odds ratio of the lowest eGFR tertile to the highest tertile was 4.21 (95% confidence interval, 1.7 to 10.43). This finding remained consistent among subgroups stratified by age, sex, diabetes status, coronary artery disease, and statin or fibrate use. CONCLUSIONS: Patients with severely reduced eGFR had higher risk of myopathy with treatment of Febuxostat. Regular monitoring of creatine kinase level is suggested for early detection of Febuxostat-associated myopathy, particularly in patients with CKD.
A Randomized Controlled Trial of the Effects of Febuxostat Therapy on Adipokines and Markers of Kidney Fibrosis in Asymptomatic Hyperuricemic Patients With Diabetic Nephropathy.[Pubmed:28270924]
Can J Kidney Health Dis. 2016 Dec 5;3:2054358116675343.
BACKGROUND: In observational studies, higher uric acid levels are associated with metabolic syndrome, diabetes, and kidney disease. OBJECTIVE: The objective of this study is to examine whether reduction of plasma uric acid with Febuxostat, a xanthine oxido reductase inhibitor, impacts adipose tissue oxidative stress, adipokines, and markers of systemic inflammation or kidney fibrosis. DESIGN: This was a double-blinded randomized controlled trial. SETTING: Academic university setting was used. PATIENTS: Overweight or obese adults with hyperuricemia and type 2 diabetic nephropathy were included. MEASUREMENTS: Adipose tissue thiobarbituric acid reducing substances (TBARS) and adiponectin concentrations and urinary transforming growth factor-beta (TGF-beta) were primary endpoints. Plasma C-reactive protein, high molecular weight-adiponectin, interleukin-6, tumor necrosis factor-alpha, and TBARS and albuminuria were among predefined secondary endpoints. METHODS: Participants were randomly assigned to Febuxostat (n = 40) or matching placebo (n = 40) and followed for 24 weeks. RESULTS: Baseline plasma uric acid levels were 426 +/- 83 micromol/L; 95% completed the study. Estimated glomerular filtration rate (eGFR) declined from 54 +/- 17 mL/min/1.73 m(2) at baseline to 51 +/- 17 mL/min/1.73 m(2) at 24 weeks (P = .05). In separate mixed-effects models, compared with placebo, Febuxostat reduced uric acid by 50% (P < .001) but had no significant effects on subcutaneous adipose tissue TBARS (-7.4%, 95% confidence interval [CI], 57.4%-101.4%) or adiponectin (6.7%, 95% CI, 26.0%-53.8%) levels or urinary TGF-beta/creatinine ratio (18.0%, 95% CI, 10.0%-54.8%) or secondary endpoints. LIMITATIONS: Relatively modest sample size and short duration of follow-up. CONCLUSIONS: In this population with progressive diabetic nephropathy, Febuxostat effectively reduced plasma uric acid. However, no detectable effects were observed for the prespecified primary or secondary endpoints. TRIAL REGISTRATION: The study was registered in clinicaltrials.gov (NCT01350388).
Xanthine oxidase inhibition with febuxostat attenuates systolic overload-induced left ventricular hypertrophy and dysfunction in mice.[Pubmed:18995179]
J Card Fail. 2008 Nov;14(9):746-53.
The purine analog xanthine oxidase (XO) inhibitors (XOIs), allopurinol and oxypurinol, have been reported to protect against heart failure secondary to myocardial infarction or rapid ventricular pacing. Because these agents might influence other aspects of purine metabolism that could influence their effect, this study examined the effect of the non-purine XOI, Febuxostat, on pressure overload-induced left ventricular (LV) hypertrophy and dysfunction. Transverse aortic constriction (TAC) in mice caused LV hypertrophy and dysfunction and increased myocardial nitrotyrosine at 8 days. TAC also caused increased phosphorylated Akt (p-Akt(Ser473)), p42/44 extracellular signal-regulated kinase (p-Erk(Thr202/Tyr204)), and mammalian target of rapamycin (mTOR) (p-mTOR(Ser2488)). XO inhibition with Febuxostat (5 mg/kg/d by gavage for 8 days) beginning approximately 60minutes after TAC attenuated the TAC-induced LV hypertrophy and dysfunction. Febuxostat blunted the TAC-induced increases in nitrotyrosine (indicating reduced myocardial oxidative stress), p-Erk(Thr202/Tyr204), and p-mTOR(Ser2488), with no effect on total Erk or total mTOR. Febuxostat had no effect on myocardial p-Akt(Ser473) or total Akt. The results suggest that XO inhibition with Febuxostat reduced oxidative stress in the pressure overloaded LV, thereby diminishing the activation of pathways that result in pathologic hypertrophy and contractile dysfunction.
An extremely potent inhibitor of xanthine oxidoreductase. Crystal structure of the enzyme-inhibitor complex and mechanism of inhibition.[Pubmed:12421831]
J Biol Chem. 2003 Jan 17;278(3):1848-55.
TEI-6720 (2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazolecarboxylic acid) is an extremely potent inhibitor of xanthine oxidoreductase. Steady state kinetics measurements exhibit mixed type inhibition with K(i) and K(i)' values of 1.2 +/- 0.05 x 10(-10) m and 9 +/- 0.05 x 10(-10) m, respectively. Fluorescence-monitored titration experiments showed that TEI-6720 bound very tightly to both the active and the inactive desulfo-form of the enzyme. The dissociation constant determined for the desulfo-form was 2 +/- 0.03 x 10(-9) m; for the active form, the corresponding number was too low to allow accurate measurements. The crystal structure of the active sulfo-form of milk xanthine dehydrogenase complexed with TEI-6720 and determined at 2.8-A resolution revealed the inhibitor molecule bound in a long, narrow channel leading to the molybdenum-pterin active site of the enzyme. It filled up most of the channel and the immediate environment of the cofactor, very effectively inhibiting the activity of the enzyme through the prevention of substrate binding. Although the inhibitor did not directly coordinate to the molybdenum ion, numerous hydrogen bonds as well as hydrophobic interactions with the protein matrix were observed, most of which are also used in substrate recognition.
Hypouricemic effect of the novel xanthine oxidase inhibitor, TEI-6720, in rodents.[Pubmed:8243554]
Eur J Pharmacol. 1993 Sep 14;241(2-3):183-8.
We investigated the xanthine oxidase/xanthine dehydrogenase inhibitory activity and hypouricemic effect of a newly synthesized xanthine oxidase/xanthine dehydrogenase inhibitor, TEI-6720, 2-(3-cyano-4-isobutoxyphenyl)-4-methyl-5-thiazole-carboxylic acid, and compared its effects with those of allopurinol in rodents. TEI-6720 was found to inhibit bovine milk xanthine oxidase, and mouse liver and rat liver xanthine oxidase/xanthine dehydrogenase with IC50 values of 1.4, 1.8 and 2.2 nM, respectively. On bovine milk xanthine oxidase, TEI-6720 exhibited mixed-type inhibition and the Ki value was 0.7 nM. TEI-6720 displayed prolonged urate-lowering activity in normal mice and rats. We evaluated the hypouricemic effect of TEI-6720 on hyperuricemia induced by the uricase inhibitor, potassium oxonate (250 mg/kg s.c., 1 h before the test drugs), and measured the total molarity of both serum allantoin and urate in rats. Oral TEI-6720 and allopurinol had a hypouricemic effect 2 h after their administration to oxonate-pretreated rats with ED50 values of 1.5 and 5.0 mg/kg, respectively. Both compounds also reduced the combined molarity of uric acid and allantoin in rats. The ED50 values of TEI-6720 and allopurinol were 2.1 and 6.9 mg/kg p.o., respectively. These results suggest that TEI-6720 may be useful for the treatment of hyperuricemia.


