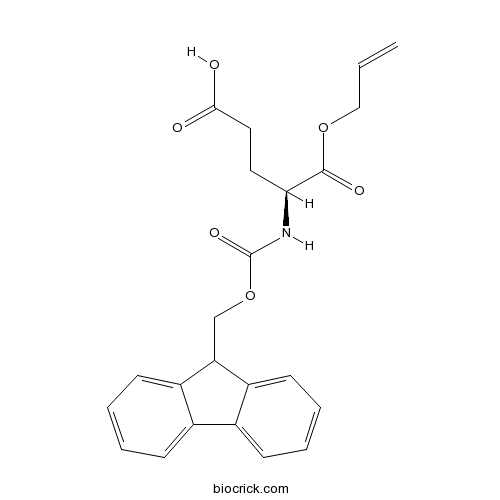
Fmoc-Glu-OAllCAS# 144120-54-7 |
- PF-4708671
Catalog No.:BCC5031
CAS No.:1255517-76-0
- BIX 02565
Catalog No.:BCC4303
CAS No.:1311367-27-7
- BI-D1870
Catalog No.:BCC5030
CAS No.:501437-28-1
- CMK
Catalog No.:BCC1489
CAS No.:821794-90-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 144120-54-7 | SDF | Download SDF |
| PubChem ID | 7020606 | Appearance | Powder |
| Formula | C23H23NO6 | M.Wt | 409.4 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | (4S)-4-(9H-fluoren-9-ylmethoxycarbonylamino)-5-oxo-5-prop-2-enoxypentanoic acid | ||
| SMILES | C=CCOC(=O)C(CCC(=O)O)NC(=O)OCC1C2=CC=CC=C2C3=CC=CC=C13 | ||
| Standard InChIKey | ORKKMGRINLTBPC-FQEVSTJZSA-N | ||
| Standard InChI | InChI=1S/C23H23NO6/c1-2-13-29-22(27)20(11-12-21(25)26)24-23(28)30-14-19-17-9-5-3-7-15(17)16-8-4-6-10-18(16)19/h2-10,19-20H,1,11-14H2,(H,24,28)(H,25,26)/t20-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Fmoc-Glu-OAll Dilution Calculator

Fmoc-Glu-OAll Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4426 mL | 12.213 mL | 24.426 mL | 48.852 mL | 61.065 mL |
| 5 mM | 0.4885 mL | 2.4426 mL | 4.8852 mL | 9.7704 mL | 12.213 mL |
| 10 mM | 0.2443 mL | 1.2213 mL | 2.4426 mL | 4.8852 mL | 6.1065 mL |
| 50 mM | 0.0489 mL | 0.2443 mL | 0.4885 mL | 0.977 mL | 1.2213 mL |
| 100 mM | 0.0244 mL | 0.1221 mL | 0.2443 mL | 0.4885 mL | 0.6106 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Fmoc-Glu-OAll
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Febuxostat
Catalog No.:BCC2556
CAS No.:144060-53-7
- 6-O-Syringoylajugol
Catalog No.:BCN6246
CAS No.:144049-72-9
- Piclamilast
Catalog No.:BCC6215
CAS No.:144035-83-6
- BRD73954
Catalog No.:BCC5652
CAS No.:1440209-96-0
- Sodium Nitroprusside
Catalog No.:BCC4844
CAS No.:14402-89-2
- Sulfapyridine
Catalog No.:BCC4729
CAS No.:144-83-2
- Sulfamethizole
Catalog No.:BCC4856
CAS No.:144-82-1
- Sulfathiazole sodium
Catalog No.:BCC5207
CAS No.:144-74-1
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Indoxacarb
Catalog No.:BCN2263
CAS No.:144171-61-9
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- YYA-021
Catalog No.:BCC5346
CAS No.:144217-65-2
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
- 1-Deazaadenosine
Catalog No.:BCC6204
CAS No.:14432-09-8
- Trilepisflavan
Catalog No.:BCN6786
CAS No.:1443218-16-3
- YM 26734
Catalog No.:BCC7396
CAS No.:144337-18-8
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
Solid-phase synthesis of peptide-4-nitroanilides.[Pubmed:8956082]
Int J Pept Protein Res. 1996 Nov;48(5):486-94.
A wide variety of Glu/Asp and Gln containing peptide-4-nitroanilides and other chromogenic peptidyl-arylamides could be quickly synthesized by a Fmoc-based solid-phase synthesis strategy employing the side-chain carboxyl groups for transient anchoring to the resin. Suitable synthons for this method, Fmoc-Glu-NH-Np and Fmoc-Asp-NH-Np, were prepared using a diphenylphosphinic chloride-mediated coupling reaction. Peptides of the common structure Suc-Ala-Phe-Pro-Xaa-NH-Np (Xaa = Glu/Asp, Gln) were synthesized and were shown to be substrates for the protease subtilisin Carlsberg (E.C.3.4.21.14a) and for peptidyl-prolyl cis/trans-isomerases (PPIases E.C. 5.2.1.8.). The method was extended to amino acids possessing a side chain missing an anchor for binding to the matrix. We synthesized Suc-Ala-Phe-Pro-Gln-Phe-NH-Np anchoring the dipeptide derivative Fmoc-Glu-Phe-NH-Np with the carboxyl group to Rink amide resin using standard SPPS procedures. Additionally this procedure allowed us the preparation of peptidyl-arylamides, utilizing the commercial available Fmoc-Glu-OAll as building block. A mixture of pentapeptide-4-nitroanilides with the general sequence Ala-Ala-Xaa-Pro-Gln-NH-Np was synthesized. Electrospray ionization mass spectrometry (ESI-MS) was used to evaluate the hydrolysis of the peptide mixture by the protease subtilisin Carlsberg. It could be shown that peptides with the hydrophobic amino acids Phe, Tyr, Leu and Val in the varied P3-position were most rapidly cleaved under the chosen conditions. Hydrolysis of the Gln-NH-Np bond in Ala-Ala-Pro-Pro-Gln-NH-Np has not been observed.


