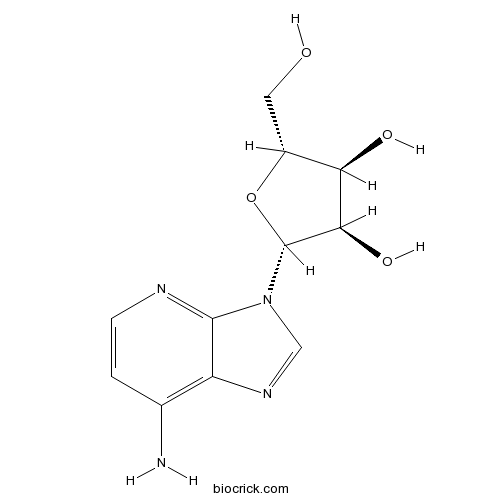
1-DeazaadenosineAdenosine deaminase inhibitor CAS# 14432-09-8 |
- CYT387 sulfate salt
Catalog No.:BCC1506
CAS No.:1056636-06-6
- Baricitinib phosphate
Catalog No.:BCC1401
CAS No.:1187595-84-1
- JAK2 Inhibitor V, Z3
Catalog No.:BCC1667
CAS No.:195371-52-9
- Bardoxolone methyl
Catalog No.:BCC1400
CAS No.:218600-53-4
- Ruxolitinib (INCB018424)
Catalog No.:BCC1276
CAS No.:941678-49-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14432-09-8 | SDF | Download SDF |
| PubChem ID | 159738 | Appearance | Powder |
| Formula | C11H14N4O4 | M.Wt | 266.25 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO | ||
| Chemical Name | (2R,3R,4S,5R)-2-(7-aminoimidazo[4,5-b]pyridin-3-yl)-5-(hydroxymethyl)oxolane-3,4-diol | ||
| SMILES | C1=CN=C2C(=C1N)N=CN2C3C(C(C(O3)CO)O)O | ||
| Standard InChIKey | NVUDDRWKCUAERS-PNHWDRBUSA-N | ||
| Standard InChI | InChI=1S/C11H14N4O4/c12-5-1-2-13-10-7(5)14-4-15(10)11-9(18)8(17)6(3-16)19-11/h1-2,4,6,8-9,11,16-18H,3H2,(H2,12,13)/t6-,8-,9-,11-/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Inhibitor of adenosine deaminase (Ki = 0.66 μM). Demonstrates antitumor activity in a range of leukemia cell lines. |

1-Deazaadenosine Dilution Calculator

1-Deazaadenosine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.7559 mL | 18.7793 mL | 37.5587 mL | 75.1174 mL | 93.8967 mL |
| 5 mM | 0.7512 mL | 3.7559 mL | 7.5117 mL | 15.0235 mL | 18.7793 mL |
| 10 mM | 0.3756 mL | 1.8779 mL | 3.7559 mL | 7.5117 mL | 9.3897 mL |
| 50 mM | 0.0751 mL | 0.3756 mL | 0.7512 mL | 1.5023 mL | 1.8779 mL |
| 100 mM | 0.0376 mL | 0.1878 mL | 0.3756 mL | 0.7512 mL | 0.939 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- ML 337
Catalog No.:BCC6345
CAS No.:1443118-44-2
- Dehydroadynerigenin glucosyldigitaloside
Catalog No.:BCN1568
CAS No.:144223-70-1
- YYA-021
Catalog No.:BCC5346
CAS No.:144217-65-2
- 1-Acetyl-4-methylpiperazine hydrochloride
Catalog No.:BCC6615
CAS No.:144205-68-5
- Indoxacarb
Catalog No.:BCN2263
CAS No.:144171-61-9
- Ledipasvir acetone
Catalog No.:BCC4046
CAS No.:1441674-54-9
- Eprosartan Mesylate
Catalog No.:BCC4658
CAS No.:144143-96-4
- Fmoc-Glu-OAll
Catalog No.:BCC3490
CAS No.:144120-54-7
- Fmoc-Asp-OAll
Catalog No.:BCC3086
CAS No.:144120-53-6
- Tarasaponin VII
Catalog No.:BCN2684
CAS No.:144118-18-3
- Deltarasin hydrochloride
Catalog No.:BCC4270
CAS No.:1440898-82-7
- Deltarasin
Catalog No.:BCC1524
CAS No.:1440898-61-2
- Trilepisflavan
Catalog No.:BCN6786
CAS No.:1443218-16-3
- YM 26734
Catalog No.:BCC7396
CAS No.:144337-18-8
- Nemoralisin C
Catalog No.:BCN7680
CAS No.:1443421-84-8
- CCG 203971
Catalog No.:BCC5601
CAS No.:1443437-74-8
- GSK2838232
Catalog No.:BCC6372
CAS No.:1443461-21-9
- 8-(7-Hydroxy-3,7-dimethyl-2,5-octadienyloxy)psoralen
Catalog No.:BCN1567
CAS No.:144398-34-5
- 3-Oxopomolic acid methyl ester
Catalog No.:BCN3723
CAS No.:14440-23-4
- Canophyllal
Catalog No.:BCN7441
CAS No.:14440-40-5
- 3-O-(E)-p-Coumaroylbetulin
Catalog No.:BCN6247
CAS No.:144424-80-6
- Aflavarin
Catalog No.:BCN7410
CAS No.:144429-67-4
- Goniodiol 8-acetate
Catalog No.:BCN4787
CAS No.:144429-71-0
- Beta-Aflatrem
Catalog No.:BCN6699
CAS No.:144446-23-1
1-Deazaadenosine: synthesis and activity of base-modified hammerhead ribozymes.[Pubmed:9461461]
Nucleic Acids Res. 1998 Feb 15;26(4):1010-8.
The incorporation of 1-Deazaadenosine (c1A, 1b) into a hammerhead ribozyme and the resulting catalytic activity is described. For this purpose the phosphoramidite 2a and the 3'-phosphonate 2b as well as Fractosil-linked 1-Deazaadenosine (3b) were prepared. The methoxyacetyl group was used for the 6-amino group protection and the triisopropylsilyl residue was introduced as the 2'-OH protecting group. Replacement of residues A14and A15.1 of the hammerhead ribozyme by 1-Deazaadenosine resulted in a significantly reduced catalytic activity. Substitution of the A6, A9 and A13 residues has only a minor influence. The findings observed on ribozymes modified with 1-Deazaadenosine were compared with those containing other adenosine analogues.
N-cycloalkyl derivatives of adenosine and 1-deazaadenosine as agonists and partial agonists of the A(1) adenosine receptor.[Pubmed:10649980]
J Med Chem. 2000 Jan 27;43(2):250-60.
A number of cycloalkyl substituents (from C-3 to C-8) have been introduced on the 6-amino group of adenosine, 1-Deazaadenosine, and 2'-deoxyadenosine, bearing or not a chlorine atom at the 2-position, to evaluate the influence on the A(1) and A(2A) affinity of steric hindrance and lipophilicity. Furthermore, the guanosine 5'-triphosphate (GTP) shift and the maximal induction of guanosine 5'-(gamma-thio)triphosphate ([(35)S]GTPgammaS) binding to G proteins in rat brain membranes were used to determine the intrinsic activity of these nucleosides at the A(1) adenosine receptor. All compounds of the ribose-bearing series proved to be full agonists, the 1-deaza derivatives showing affinities for the A(1) receptor about 10-fold lower than the corresponding adenosines. On the other hand, all the 2'-deoxyribose derivatives bind to the A(1) receptor with affinities in the high nanomolar range, with the 2-chloro substituted compounds showing slightly higher affinities than the 2-unsubstituted counterparts. In terms of the potencies, the most potent compounds proved to be those bearing four- and five-membered rings. Both GTP shifts and [(35)S]-GTPgammaS experiments showed that most of the 2'-deoxyadenosine derivatives are partial agonists. The 2'-deoxyadenosine derivatives which were identified as partial agonists consistently detected fewer A(1) receptors in the high-affinity state than full agonists. However, it is worthwhile noting that there was not a simple linear relationship between receptor occupancy and activation. These results indicate that a critical density of A(1) adenosine receptors in the high-affinity state is required for G protein activation.
2-Nitro analogues of adenosine and 1-deazaadenosine: synthesis and binding studies at the adenosine A1, A2A and A3 receptor subtypes.[Pubmed:10999489]
Bioorg Med Chem Lett. 2000 Sep 18;10(18):2141-4.
The influence of nitro substituents on the properties of adenosine and 1-Deazaadenosine was studied. Combination of a nitro group at the 2-position with several N6 substituents such as cyclopentyl and m-iodobenzyl gave a series of analogues with good adenosine receptor affinity, showing directable selectivity for the A1, A2A and A3 adenosine receptor subtypes.
Binding thermodynamics of the transition state analogue coformycin and of the ground state analogue 1-deazaadenosine to bovine adenosine deaminase.[Pubmed:11697042]
J Enzyme Inhib. 2001;16(3):217-32.
Binding of the transition state analogue coformycin and the ground state analogue 1-deaazadenosine to bovine adenosine deaminase have been thermodynamically characterized. The heat capacity changes for coformycin and 1-Deazaadenosine binding are -4.7 +/- 0.8 kJ/mole-K and -1.2 +/- 0.1 kJ/mole-K, respectively. Since the predominant source of heat capacity change in enzyme interactions are changes in the extent of exposure of nonpolar amino acid side chains to the aqueous environment and the hydrophobic effect is the predominant factor in native structure stabilization, we propose that the binding of either class of ligand is associated with a stabilizing enzyme conformational change with coformycin producing the far greater effect. Analysis of the T dependence of the second order rate constant for formation of the enzyme/coformycin complex further reveals that the conformational change is not rate limiting. We propose that the enzyme may facilitate catalysis via the formation of a stabilizing conformation at the reaction transition state.
Improved synthesis and antitumor activity of 1-deazaadenosine.[Pubmed:3625714]
J Med Chem. 1987 Sep;30(9):1686-8.
A more convenient synthetic route to 1-Deazaadenosine (1) by reduction of the new nucleoside 7-nitro-3-beta-D-ribofuranosyl-3H-imidazo[4,5-b]pyridine (6) is reported. Compound 6 was obtained by reaction of 7-nitroimidazo-[4,5-b]pyridine with 1,2,3,5-tetra-O-acetyl-beta-D-ribofuranose in the presence of stannic chloride followed by treatment with methanolic ammonia. 1-Deazaadenosine (1) showed good activity in vitro as inhibitor of HeLa, KB, P388, and L1210 leukemia cell line growth, with ID50 values ranging from 0.34 microM (KB) to 1.8 microM (P388). The nitro derivative 6 demonstrated moderate activity against the same cell lines.


